Abstract
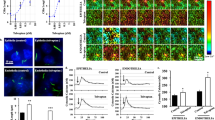
Dysfunction of many ciliary proteins has been linked to a list of diseases, from cystic kidney to obesity and from hypertension to mental retardation. We previously proposed that primary cilia are unique communication organelles that function as microsensory compartments that house mechanosensory molecules. Here we report that primary cilia exhibit membrane swellings or ciliary bulbs, which based on their unique ultrastructure and motility, could be mechanically regulated by fluid-shear stress. Together with the ultrastructure analysis of the swelling, which contains monosialodihexosylganglioside (GM3), our results show that ciliary bulb has a distinctive set of functional proteins, including GM3 synthase (GM3S), bicaudal-c1 (Bicc1), and polycystin-2 (PC2). In fact, results from our cilia isolation demonstrated for the first time that GM3S and Bicc1 are members of the primary cilia proteins. Although these proteins are not required for ciliary membrane swelling formation under static condition, fluid-shear stress induced swelling formation is partially modulated by GM3S. We therefore propose that the ciliary bulb exhibits a sensory function within the mechano-ciliary structure. Overall, our studies provided an important step towards understanding the ciliary bulb function and structure.









Similar content being viewed by others
References
Zimmermann K (1898) Beiträge zur Kenntnis einiger Drüsen und Epithelien. Arch Mikroskop Anat 52:552–706
Tobin JL, Beales PL (2009) The nonmotile ciliopathies. Genetics Med 11(6):386–402. doi:10.1097/GIM.0b013e3181a02882
Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33(2):129–137. doi:10.1038/ng1076
Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J (2008) Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117(9):1161–1171. doi:10.1161/CIRCULATIONAHA.107.710111
Praetorius HA, Spring KR (2001) Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184(1):71–79
Jin X, Mohieldin AM, Muntean BS, Green JA, Shah JV, Mykytyn K, Nauli SM (2014) Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol Life Sci 71(11):2165–2178. doi:10.1007/s00018-013-1483-1
Aboualaiwi WA, Muntean BS, Ratnam S, Joe B, Liu L, Booth RL, Rodriguez I, Herbert BS, Bacallao RL, Fruttiger M, Mak TW, Zhou J, Nauli SM (2014) Survivin-induced abnormal ploidy contributes to cystic kidney and aneurysm formation. Circulation 129(6):660–672. doi:10.1161/CIRCULATIONAHA.113.005746
AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM (2009) Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res 104(7):860–869. doi:10.1161/CIRCRESAHA.108.192765
Takaoki M, Murakami N, Gyotoku J (2004) C-fos expression of osteoblast-like MC3T3-E1 cells induced either by cooling or by fluid flow. Uchu Seibutsu Kagaku 18(3):181–182
McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA (2007) Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol 26(4):234–246. doi:10.1016/j.matbio.2006.12.003
Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, Bowser SS (2004) Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int 28(2):101–110. doi:10.1016/j.cellbi.2003.11.007
Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF (2006) Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131(3):911–920. doi:10.1053/j.gastro.2006.07.003
Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, Nakai J, Dworniczak B, Ehrlich BE, Pennekamp P, Hamada H (2012) Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 338(6104):226–231. doi:10.1126/science.1222538
McGrath J, Somlo S, Makova S, Tian X, Brueckner M (2003) Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114(1):61–73
Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T (2013) Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods 10(11):1105–1107. doi:10.1038/nmeth.2647
Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H (2010) Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol 298(5):F1096–F1102. doi:10.1152/ajprenal.00657.2009
Xu C, Rossetti S, Jiang L, Harris PC, Brown-Glaberman U, Wandinger-Ness A, Bacallao R, Alper SL (2007) Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+signaling. Am J Physiol Renal Physiol 292(3):F930–F945. doi:10.1152/ajprenal.00285.2006
Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J (2006) Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol 17(4):1015–1025. doi:10.1681/ASN.2005080830
Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD (2006) Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol 290(6):F1320–F1328. doi:10.1152/ajprenal.00463.2005
Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM (2005) Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289(5):F978–F988. doi:10.1152/ajprenal.00260.2004
Cano DA, Sekine S, Hebrok M (2006) Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology 131(6):1856–1869. doi:10.1053/j.gastro.2006.10.050
Cano DA, Murcia NS, Pazour GJ, Hebrok M (2004) Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development 131(14):3457–3467. doi:10.1242/dev.01189
Hoey DA, Tormey S, Ramcharan S, O’Brien FJ, Jacobs CR (2012) Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells 30(11):2561–2570. doi:10.1002/stem.1235
Gilula NB, Satir P (1972) The ciliary necklace. A ciliary membrane specialization. J Cell Biol 53(2):494–509
Dilly PN (1977) Further observations of transport within paddle cilia. Cell Tissue Res 185(1):105–113
Dilly PN (1977) Material transport within specialised ciliary shafts on Rhabdopleura zooids. Cell Tissue Res 180(3):367–381
Beninger PG, Potter TM, Stjean SD (1995) Paddle cilia fixation artifacts in pallial organs of adult Mytilus edulis and Placopecten magennanicus. Canadian J Zool 73:610–614
Short G, Tamm SL (1991) On the nature of paddle cilia and discocilia. Biol Bull 180:466–474
Menco BP, Farbman AI (1985) Genesis of cilia and microvilli of rat nasal epithelia during pre-natal development. II. Olfactory epithelium, a morphometric analysis. J Cell Sci 78:311–336
Menco BP, Farbman AI (1985) Genesis of cilia and microvilli of rat nasal epithelia during pre-natal development. I. Olfactory epithelium, qualitative studies. J Cell Sci 78:283–310
Holley A, Mac Leod P (1977) Transduction and coding of olfactory information. Journal de physiologie 73(6):725–848
Roth KE, Rieder CL, Bowser SS (1988) Flexible-substratum technique for viewing cells from the side: some in vivo properties of primary (9 + 0) cilia in cultured kidney epithelia. J Cell Sci 89(Pt 4):457–466
Janich P, Corbeil D (2007) GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett 581(9):1783–1787. doi:10.1016/j.febslet.2007.03.065
Yoder BK, Hou X, Guay-Woodford LM (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13(10):2508–2516
Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB (2002) Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12(11):R378–R380
Tran U, Zakin L, Schweickert A, Agrawal R, Doger R, Blum M, De Robertis EM, Wessely O (2010) The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development 137(7):1107–1116. doi:10.1242/dev.046045
Mitchell KA (2013) Isolation of primary cilia by shear force. Current protocols in cell biology/editorial board, Juan S Bonifacino [et al] Chapter 3:Unit 3 42 41–49. doi:10.1002/0471143030.cb0342s59
Berselli P, Zava S, Sottocornola E, Milani S, Berra B, Colombo I (2006) Human GM3 synthase: a new mRNA variant encodes an NH2-terminal extended form of the protein. Biochim Biophys Acta 1759(7):348–358. doi:10.1016/j.bbaexp.2006.07.001
Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S (2002) Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4(3):191–197. doi:10.1038/ncb754
Elofsson R, Andersson A, Falck B, Sjoborg S (1984) The ciliated human keratinocyte. J Ultrastruct Res 87(3):212–220
Dalen H (1981) An ultrastructural study of primary cilia, abnormal cilia and ciliary knobs from the ciliated cells of the guinea-pig trachea. Cell Tissue Res 220(4):685–697
Albrecht-Buehler G, Bushnell A (1980) The ultrastructure of primary cilia in quiescent 3T3 cells. Exp Cell Res 126(2):427–437
Jensen CG, Davison EA, Bowser SS, Rieder CL (1987) Primary cilia cycle in PtK1 cells: effects of colcemid and taxol on cilia formation and resorption. Cell Motil Cytoskelet 7(3):187–197. doi:10.1002/cm.970070302
Jensen CG, Jensen LC, Rieder CL (1979) The occurrence and structure of primary cilia in a subline of Potorous tridactylus. Exp Cell Res 123(2):444–449
Wood CR, Huang K, Diener DR, Rosenbaum JL (2013) The cilium secretes bioactive ectosomes. Curr Biol 23(10):906–911. doi:10.1016/j.cub.2013.04.019
Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, Charlesworth MC, Torres VE, LaRusso NF, Harris PC, Ward CJ (2009) Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 20(2):278–288. doi:10.1681/ASN.2008060564
Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH, Barr MM (2014) C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol 24(5):519–525. doi:10.1016/j.cub.2014.01.002
Kaimori JY, Nagasawa Y, Menezes LF, Garcia-Gonzalez MA, Deng J, Imai E, Onuchic LF, Guay-Woodford LM, Germino GG (2007) Polyductin undergoes notch-like processing and regulated release from primary cilia. Hum Mol Genet 16(8):942–956. doi:10.1093/hmg/ddm039
Hidaka S, Konecke V, Osten L, Witzgall R (2004) PIGEA-14, a novel coiled-coil protein affecting the intracellular distribution of polycystin-2. J Biol Chem 279(33):35009–35016. doi:10.1074/jbc.M314206200
Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S (1999) Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem 274(40):28557–28565
Wang L, Eckmann CR, Kadyk LC, Wickens M, Kimble J (2002) A regulatory cytoplasmic poly(A) polymerase in Caenorhabditis elegans. Nature 419(6904):312–316. doi:10.1038/nature01039
Saffman EE, Styhler S, Rother K, Li W, Richard S, Lasko P (1998) Premature translation of oskar in oocytes lacking the RNA-binding protein bicaudal-C. Mol Cell Biol 18(8):4855–4862
Mahone M, Saffman EE, Lasko PF (1995) Localized Bicaudal-C RNA encodes a protein containing a KH domain, the RNA binding motif of FMR1. EMBO J 14(9):2043–2055
Acknowledgments
This work partially fulfills the requirements of a PhD degree in Medicinal and Biological Chemistry for Ashraf M. Mohieldin. This work was supported by the Department of Defense PR130153 (SMN) and in part by the National Institute of Health R01DK080640 (SMN), R01DK080745 (OW) and F31DK096870 (AMM).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 8 (AVI 553 kb)
Supplementary material 9 (AVI 1498 kb)
Rights and permissions
About this article
Cite this article
Mohieldin, A.M., Haymour, H.S., Lo, S.T. et al. Protein composition and movements of membrane swellings associated with primary cilia. Cell. Mol. Life Sci. 72, 2415–2429 (2015). https://doi.org/10.1007/s00018-015-1838-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-1838-x