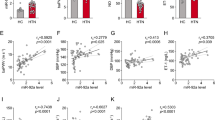
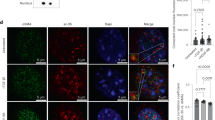
Abstract
Laminin of different cellular sources has distinct functions. In addition to vascular smooth muscle cells (SMCs), aorta also contains a small population of nestin+ cells, whose function remains unknown. This study investigates the role of SMC- and nestin+ cell-derived laminin in blood pressure (BP) regulation and SMC contractibility. Using mice with laminin deficiency in SMCs (SKO) or nestin+ cells (NKO), we examined laminin-dependent changes in BP. Contractile protein expression was reduced in SKO but not NKO mice, consistent with their, respectively, low and normal baseline BP measurements. At the ultrastructural level, SKO SMCs maintained the contractile phenotype with reduced elasticity, whereas NKO SMCs switched to the synthetic phenotype and showed degeneration. Additionally, angiotensin II (Ang II) significantly increased BP in SKO but not NKO mice. It also enhanced contractile proteins to the same levels and induced SMC degeneration in both knockout mice. These data suggest that SMC laminin regulates BP via modulating contractile protein expression, whereas nestin+ cell-derived laminin contributes to SMC phenotypic switch.








Similar content being viewed by others
Abbreviations
- BP:
-
Blood pressure
- Ctr:
-
Control
- F/F:
-
Laminin γ1flox/flox
- SKO:
-
Laminin γ1flox/flox: SM22α-cre+
- NKO:
-
Laminin γ1flox/flox: nestin-cre+
- SMC:
-
Smooth muscle cells
- SMA:
-
Smooth muscle actin-α
- Ang II:
-
Angiotensin II
- ATR1:
-
Angiotensin II receptor type-I
- ATR2:
-
Angiotensin II receptor type-II
- ECM:
-
Extracellular matrix
- EL:
-
Elastin laminae
References
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D (2012) Turner MB Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125(1):e2–e220
Perry RL, Rudnick MA (2000) Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci 5:D750–D767
Davis-Dusenbery BN, Wu C, Hata A (2011) Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler Thromb Vasc Biol 31(11):2370–2377
Gomez D, Owens GK (2012) Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 95(2):156–164
Alexander MR, Owens GK (2012) Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 74:13–40. doi:10.1146/annurev-physiol-012110-142315
Welser JV, Lange N, Singer CA, Elorza M, Scowen P, Keef KD, Gerthoffer WT, Burkin DJ (2007) Loss of the alpha7 integrin promotes extracellular signal-regulated kinase activation and altered vascular remodeling. Circ Res 101(7):672–681
Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, DE Vatner, Meininger GA (2010) Vatner SF Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res 107(5):615–619
Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB (2012) The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res 95(2):194–204
Jacob MP, Badier-Commander C, Fontaine V, Benazzoug Y, Feldman L, Michel JB (2001) Extracellular matrix remodeling in the vascular wall. Pathol Biol (Paris) 49(4):326–332
Chen ZL, Yao Y, Norris EH, Kruyer A, Jno-Charles O, Akhmerov A, Strickland S (2013) Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J Cell Biol 202(2):381–395
Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ (2000) Impaired vascular contractility and blood pressure homeostasis in the smooth muscle alpha-actin null mouse. FASEB J 14(14):2213–2220
Tran T, Teoh CM, Tam JK, Qiao Y, Chin CY, Chong OK, Stewart AG, Harris T, Wong WS, Guan SP, Leung BP, Gerthoffer WT, Unruh H, Halayko AJ (2013) Laminin drives survival signals to promote a contractile smooth muscle phenotype and airway hyperreactivity. FASEB J 27(10):3991–4003
Hultgardh-Nilsson A, Durbeej M (2007) Role of the extracellular matrix and its receptors in smooth muscle cell function: implications in vascular development and disease. Curr Opin Lipidol 18(5):540–545
Thyberg J, Hultgardh-Nilsson A (1994) Fibronectin and the basement membrane components laminin and collagen type IV influence the phenotypic properties of subcultured rat aortic smooth muscle cells differently. Cell Tissue Res 276(2):263–271
Oikawa H, Hayashi K, Maesawa C, Masuda T, Sobue K (2010) Expression profiles of nestin in vascular smooth muscle cells in vivo and in vitro. Exp Cell Res 316(6):940–950
Flanagan LA, Rebaza LM, Derzic S, Schwartz PH, Monuki ES (2006) Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res 83(5):845–856
Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD (2010) Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab 30(1):56–69
Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K (2008) Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21(12):1288–1291
Yao Y, Chen ZL, Norris EH, Strickland S (2014) Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun 5:3413
Pang SC, Scott TM (1981) Stereological analysis of the tunica media of the aorta and renal artery during the development of hypertension in the spontaneously hypertensive rat. J Anat 133(Pt 4):513–526
Popescu MR, Zugun FE, Cojocaru E, Tocan L, Folescu R, Zamfir CL (2013) Morphometric study of aortic wall parameters evolution in newborn and child. Rom J Morphol Embryol 54(2):399–404
Kochova P, Tonar Z, Matejka VM, Sviglerova J, Stengl M, Kuncova J (2008) Morphology and mechanical properties of the subrenal aorta in normotensive and hypertensive rats. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 152(2):239–245
Schmidt VJ, Jobs A, von Maltzahn J, Worsdorfer P, Willecke K, de Wit C (2012) Connexin45 is expressed in vascular smooth muscle but its function remains elusive. PLoS One 7(7):e42287
Heeneman S, Sluimer JC, Daemen MJ (2007) Angiotensin-converting enzyme and vascular remodeling. Circ Res 101(5):441–454
Li J, Jiang J, Yin H, Wang L, Tian R, Li H, Wang Z, Li D, Wang Y, Gui Y, Walsh MP, Zheng XL (2012) Atorvastatin inhibits myocardin expression in vascular smooth muscle cells. Hypertension 60(1):145–153
Han M, Dong LH, Zheng B, Shi JH, Wen JK, Cheng Y (2009) Smooth muscle 22 alpha maintains the differentiated phenotype of vascular smooth muscle cells by inducing filamentous actin bundling. Life Sci 84(13–14):394–401
Rensen SS, Doevendans PA, van Eys GJ (2007) Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 15(3):100–108
Wanjare M, Kusuma S, Gerecht S (2014) Defining differences among perivascular cells derived from human pluripotent stem cells. Stem Cell Reports 2(5):561–575
Rindler TN, Dostanic I, Lasko VM, Nieman ML, Neumann JC, Lorenz JN, Lingrel JB (2011) Knockout of the Na, K-ATPase alpha(2)-isoform in the cardiovascular system does not alter basal blood pressure but prevents ACTH-induced hypertension. Am J Physiol Heart Circ Physiol 301(4):H1396–H1404
Lepore JJ, Cheng L, Min LuM, Mericko PA, Morrisey EE, Parmacek MS (2005) High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22alpha-Cre transgenic mice. Genesis 41(4):179–184
Hassane S, Claij N, Jodar M, Dedman A, Lauritzen I, Duprat F, Koenderman JS, van der Wal A, Breuning MH, de Heer E, Honore E, DeRuiter MC, Peters DJ (2011) Pkd1-inactivation in vascular smooth muscle cells and adaptation to hypertension. Lab Invest 91(1):24–32
Dubois NC, Hofmann D, Kaloulis K, Bishop JM, Trumpp A (2006) Nestin-Cre transgenic mouse line Nes-Cre1 mediates highly efficient Cre/loxP mediated recombination in the nervous system, kidney, and somite-derived tissues. Genesis 44(8):355–360
Majesky MW (2007) Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27(6):1248–1258
Rosenquist TH, Kirby ML, van Mierop LH (1989) Solitary aortic arch artery. A result of surgical ablation of cardiac neural crest and nodose placode in the avian embryo. Circulation 80(5):1469–1475
Topouzis S, Majesky MW (1996) Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Dev Biol 178(2):430–445
Madura JA 2nd, Kaufman BR, Margolin DA, Spencer DM, Fox PL, Graham LM (1996) Regional differences in platelet-derived growth factor production by the canine aorta. J Vasc Res 33(1):53–61
Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM (2001) Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol 153(5):933–946
Yousif LF, Di Russo J, Sorokin L (2013) Laminin isoforms in endothelial and perivascular basement membranes. Cell Adh Migr 7(1):101–110
Jucker M, Tian M, Norton DD, Sherman C, Kusiak JW (1996) Laminin alpha 2 is a component of brain capillary basement membrane: reduced expression in dystrophic dy mice. Neuroscience 71(4):1153–1161
Sorokin LM, Pausch F, Frieser M, Kroger S, Ohage E, Deutzmann R (1997) Developmental regulation of the laminin alpha5 chain suggests a role in epithelial and endothelial cell maturation. Dev Biol 189(2):285–300
Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM (2005) Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev 85(3):979–1000
Rauch U, Saxena A, Lorkowski S, Rauterberg J, Bjorkbacka H, Durbeej M, Hultgardh-Nilsson A (2011) Laminin isoforms in atherosclerotic arteries from mice and man. Histol Histopathol 26(6):711–724
Acknowledgments
We thank Dr. Kunihiro Uryu for assistance with electron microscopy and members of the Strickland Laboratory for scientific discussions. This work was supported by NIH grant NS050537 (SS), a Merck Postdoctoral Fellowship (YY), and a BD Stem Cell Grant (YY).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2014_1732_MOESM1_ESM.tif
Supplementary material 1 Laminin γ1 expression in SMCs and nestin+ cells in Ctr, SKO, and NKO aortas. Laminin γ1 (Ln-γ1, red) is absent in SM22α+ (dark brown-black) SMCs and nestin+ (Alexa-647, artificially labeled in green) cells in SKO and NKO aortas, respectively. Blue arrows indicate SM22α+ SMCs. White arrowheads indicate nestin+ cells. Scale bar represents 45 μm. (TIFF 6330 kb)
18_2014_1732_MOESM2_ESM.tif
Supplementary material 2 Loss of laminin induces proliferation in aorta. Ki67 (dark brown-black) co-localizes with some SMA+ (red) SMCs in SKO aorta, and both SMA+ (red) and nestin+ (Alexa-647, artificially labeled in green) cells in NKO aorta. Blue arrows indicate SMA+ SMCs in Ctr aorta. Blue arrowheads indicate SMA+Ki67+ SMCs in SKO aorta. White arrows and white arrowheads indicate nestin+Ki67+ and SMA+Ki67+ cells in NKO aorta, respectively. Scale bar represents 45 μm. (TIFF 6466 kb)
18_2014_1732_MOESM3_ESM.tif
Supplementary material 3 Nestin+ cells are mesenchymal stem cells. Nestin+ cells (dark brown-black) in aorta express CD90 (red), CD105 (red), PDGFRα (red), and c-Kit (red). Scale bar represents 20 μm. (TIFF 18537 kb)
18_2014_1732_MOESM4_ESM.tif
Supplementary material 4 Electron microscopic images of aorta at low magnification. High resolution electron microscopic images of Ctr, SKO, and NKO aortas before and after Ang II treatment. Scale bar represents 2 μm.(TIFF 34835 kb)
18_2014_1732_MOESM5_ESM.tif
Supplementary material 5 Calponin is down-regulated in SKO aorta. (a) Confocal images of calponin expression in Ctr, SKO, and NKO aortas. (b) Representative western blots and semi-quantification of calponin expression in Ctr, SKO, and NKO aortas. All bands were normalized to actin. Data are shown as mean ± SD, n = 4. *p < 0.05 versus Ctr. Scale bar represents 20 μm.(TIFF 8943 kb)
18_2014_1732_MOESM6_ESM.tif
Supplementary material 6 SMA and SM22α expression is reduced in resistance arteries in SKO mice. (a) SMA (green) and CD31 (red) staining in resistance arteries in Ctr, SKO, and NKO mice. (b) Quantification of SMA and SM22α expression levels. Data are shown as mean ± SD, n = 4-5. **p < 0.01 versus Ctr. Scale bar represents 100 μm. (TIFF 8953 kb)
18_2014_1732_MOESM7_ESM.tif
Supplementary material 7 Body weight changes during Ang II treatment. Body weight of Ctr, SKO, and NKO mice at different time points after Ang II treatment. Data are shown as mean ± SD, n = 4. (TIFF 2261 kb)
Rights and permissions
About this article
Cite this article
Yao, Y., Norris, E.H. & Strickland, S. The cellular origin of laminin determines its role in blood pressure regulation. Cell. Mol. Life Sci. 72, 999–1008 (2015). https://doi.org/10.1007/s00018-014-1732-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1732-y