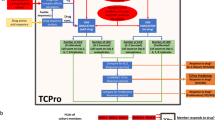
Abstract
Biotherapeutics are subject to immune surveillance within the body, and anti-biotherapeutic immune responses can compromise drug efficacy and patient safety. Initial development of targeted antidrug immune memory is coordinated by T cell recognition of immunogenic subsequences, termed “T cell epitopes.” Biotherapeutics may therefore be deimmunized by mutating key residues within cognate epitopes, but there exist complex trade-offs between immunogenicity, mutational load, and protein structure–function. Here, a protein deimmunization algorithm has been applied to P99 beta-lactamase, a component of antibody-directed enzyme prodrug therapies. The algorithm, integer programming for immunogenic proteins, seamlessly integrates computational prediction of T cell epitopes with both 1- and 2-body sequence potentials that assess protein tolerance to epitope-deleting mutations. Compared to previously deimmunized P99 variants, which bore only one or two mutations, the enzymes designed here contain 4–5 widely distributed substitutions. As a result, they exhibit broad reductions in major histocompatibility complex recognition. Despite their high mutational loads and markedly reduced immunoreactivity, all eight engineered variants possessed wild-type or better catalytic activity. Thus, the protein design algorithm is able to disrupt broadly distributed epitopes while maintaining protein function. As a result, this computational tool may prove useful in expanding the repertoire of next-generation biotherapeutics.





Similar content being viewed by others
Abbreviations
- APCs:
-
Antigen-presenting cells
- MHC II:
-
Class II major histocompatibility complex proteins
- DP2 :
-
Dynamic programming for deimmunizing proteins
- P99βL:
-
Enterobacter chloacae P99 beta-lactamase
- IP2 :
-
Integer programming for immunogenic proteins
References
Baker MP, Reynolds HM, Lumicisi B, Bryson CJ (2010) Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self Nonself 1(4):314–322. doi:10.4161/self.1.4.13904
Barbosa MD (2011) Immunogenicity of biotherapeutics in the context of developing biosimilars and biobetters. Drug Discov Today 16(7–8):345–353. doi:10.1016/j.drudis.2011.01.011
De Groot AS, Scott DW (2007) Immunogenicity of protein therapeutics. Trends Immunol 28(11):482–490. doi:10.1016/J.It.2007.07.011
Schellekens H (2007) Immunogenicity of protein therapeutics, or how to make antibodies without T-cells. Inflamm Res 56:S351–S352
Schellekens H (2005) Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant 20(Suppl 6):vi3–vi9. doi:10.1093/ndt/gfh1092
Trombetta ES, Mellman I (2005) Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 23:975–1028. doi:10.1146/annurev.immunol.22.012703.104538
Warmerdam PA, Plaisance S, Vanderlick K, Vandervoort P, Brepoels K, Collen D, De Maeyer M (2002) Elimination of a human T-cell region in staphylokinase by T-cell screening and computer modeling. Thromb Haemost 87(4):666–673
Jones TB, Hart RP, Popovich PG (2005) Molecular control of physiological and pathological T-cell recruitment after mouse spinal cord injury. J Neurosci 25(28):6576–6583. doi:10.1523/JNEUROSCI.0305-05.2005
Harding FA, Liu AD, Stickler M, Razo OJ, Chin R, Faravashi N, Viola W, Graycar T, Yeung VP, Aehle W, Meijer D, Wong S, Rashid MH, Valdes AM, Schellenberger V (2005) A beta-lactamase with reduced immunogenicity for the targeted delivery of chemotherapeutics using antibody-directed enzyme prodrug therapy. Mol Cancer Ther 4(11):1791–1800. doi:10.1158/1535-7163.MCT-05-0189
Cizeau J, Grenkow DM, Brown JG, Entwistle J, MacDonald GC (2009) Engineering and biological characterization of VB6-845, an anti-EpCAM immunotoxin containing a T-cell epitope-depleted variant of the plant toxin bouganin. J Immunother 32(6):574–584. doi:10.1097/CJI.0b013e3181a6981c
Singh H, Raghava GP (2001) ProPred: prediction of HLA-DR binding sites. Bioinformatics 17(12):1236–1237
Guan P, Doytchinova IA, Zygouri C, Flower DR (2003) MHCPred: bringing a quantitative dimension to the online prediction of MHC binding. Appl Bioinform 2(1):63–66
Wan J, Liu W, Xu Q, Ren Y, Flower DR, Li T (2006) SVRMHC prediction server for MHC-binding peptides. BMC Bioinform 7:463. doi:10.1186/1471-2105-7-463
Bui HH, Sidney J, Peters B, Sathiamurthy M, Sinichi A, Purton KA, Mothe BR, Chisari FV, Watkins DI, Sette A (2005) Automated generation and evaluation of specific MHC binding predictive tools: ARB matrix applications. Immunogenetics 57(5):304–314. doi:10.1007/s00251-005-0798-y
Nielsen M, Lundegaard C, Lund O (2007) Prediction of MHC class II binding affinity using SMM-align, a novel stabilization matrix alignment method. BMC Bioinform 8:238. doi:10.1186/1471-2105-8-238
Nielsen M, Justesen S, Lund O, Lundegaard C, Buus S (2010) NetMHCIIpan-2.0—Improved pan-specific HLA-DR predictions using a novel concurrent alignment and weight optimization training procedure. Immunome Res 6:9. doi:10.1186/1745-7580-6-9
Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B (2008) A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol 4(4):e1000048. doi:10.1371/journal.pcbi.1000048
De Groot AS, Knopp PM, Martin W (2005) De-immunization of therapeutic proteins by T-cell epitope modification. Dev Biol (Basel) 122:171–194
Perry LC, Jones TD, Baker MP (2008) New approaches to prediction of immune responses to therapeutic proteins during preclinical development. Drugs R D 9(6):385–396. doi:10.2165/0126839-200809060-00004
De Groot AS, Martin W (2009) Reducing risk, improving outcomes: bioengineering less immunogenic protein therapeutics. Clin Immunol 131(2):189–201. doi:10.1016/j.clim.2009.01.009
Kern F, LiPira G, Gratama JW, Manca F, Roederer M (2005) Measuring Ag-specific immune responses: understanding immunopathogenesis and improving diagnostics in infectious disease, autoimmunity and cancer. Trends Immunol 26(9):477–484. doi:10.1016/j.it.2005.07.005
Li Pira G, Ivaldi F, Moretti P, Manca F (2010) High throughput T epitope mapping and vaccine development. J Biomed Biotechnol 2010:325720. doi:10.1155/2010/325720
Salvat RS, Moise L, Bailey-Kellogg C, Griswold KE (2014) A high throughput MHC II binding assay for quantitative analysis of peptide epitopes. J Vis Exp (in press). doi:10.1093/protein/gzs044
Moise L, Song C, Martin WD, Tassone R, De Groot AS, Scott DW (2012) Effect of HLA DR epitope de-immunization of Factor VIII in vitro and in vivo. Clin Immunol 142(3):320–331. doi:10.1016/j.clim.2011.11.010
Cantor JR, Yoo TH, Dixit A, Iverson BL, Forsthuber TG, Georgiou G (2011) Therapeutic enzyme deimmunization by combinatorial T-cell epitope removal using neutral drift. Proc Natl Acad Sci USA 108(4):1272–1277. doi:10.1073/pnas.1014739108
Koren E, De Groot AS, Jawa V, Beck KD, Boone T, Rivera D, Li L, Mytych D, Koscec M, Weeraratne D, Swanson S, Martin W (2007) Clinical validation of the “in silico” prediction of immunogenicity of a human recombinant therapeutic protein. Clin Immunol 124(1):26–32. doi:10.1016/j.clim.2007.03.544
Parker AS, Zheng W, Griswold KE, Bailey-Kellogg C (2010) Optimization algorithms for functional deimmunization of therapeutic proteins. BMC Bioinform 11:180. doi:10.1186/1471-2105-11-180
Parker AS, Griswold KE, Bailey-Kellogg C (2011) Optimization of therapeutic proteins to delete T-cell epitopes while maintaining beneficial residue interactions. J Bioinform Comput Biol 9(2):207–229
Parker AS, Choi Y, Griswold KE, Bailey-Kellogg C (2013) Structure-guided deimmunization of therapeutic proteins. J Comput Biol 20(2):152–165. doi:10.1089/cmb.2012.0251
Choi Y, Griswold Ke, Bailey-Kellogg C Structure-based redesign of proteins for minimal T-cell epitope content. (1096-987X (Electronic))
Osipovitch DC, Parker AS, Makokha CD, Desrosiers J, Kett WC, Moise L, Bailey-Kellogg C, Griswold KE (2012) Design and analysis of immune-evading enzymes for ADEPT therapy. Protein Eng Des Sel 25(10):613–623. doi:10.1093/protein/gzs044
Sandler I, Zigdon N, Levy E, Aharoni A (2014) The functional importance of co-evolving residues in proteins. Cell Mol Life Sci 71:673–682
McCaldon P, Argos P (1988) Oligopeptide biases in protein sequences and their use in predicting protein coding regions in nucleotide-sequences. Proteins Struct Funct Genet 4(2):99–122. doi:10.1002/prot.340040204
Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, Kubo RT, Chesnut RW, Grey HM, Sette A (1998) Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol 160(7):3363–3373
Stemmer WP, Crameri A, Ha KD, Brennan TM, Heyneker HL (1995) Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene 164(1):49–53
Mazor R, Vassall AN, Eberle JA, Beers R, Weldon JE, Venzon DJ, Tsang KY, Benhar I, Pastan I (2012) Identification and elimination of an immunodominant T-cell epitope in recombinant immunotoxins based on Pseudomonas exotoxin A. Proc Natl Acad Sci 109(51):E3597–E3603. doi:10.1073/pnas.1218138109
Yeung VP, Chang J, Miller J, Barnett C, Stickler M, Harding FA (2004) Elimination of an immunodominant CD4+ T cell epitope in human IFN-β does not result in an in vivo response directed at the subdominant epitope. J Immunol 172(11):6658–6665
Tangri S, Mothe BR, Eisenbraun J, Sidney J, Southwood S, Briggs K, Zinckgraf J, Bilsel P, Newman M, Chesnut R, LiCalsi C, Sette A (2005) Rationally engineered therapeutic proteins with reduced immunogenicity. J Immunol 174(6):3187–3196
Onda M (2009) Reducing the immunogenicity of protein therapeutics. Curr Drug Targets 10(2):131–139
Lee S (2010) Implications of available design space for identification of non-immunogenic protein therapeutics. Biomed Microdevices 12(2):283–286. doi:10.1007/s10544-009-9383-8
Hill JA, Wang DQ, Jevnikar AM, Cairns E, Bell DA (2003) The relationship between predicted peptide-MHC class II affinity and T-cell activation in a HLA-DR beta 1*0401 transgenic mouse model. Arthrit Res Therapy 5(1):R40–R48. doi:10.1186/ar605
Weaver JM, Sant AJ (2009) Understanding the focused CD4 T cell response to antigen and pathogenic organisms. Immunol Res 45(2–3):123–143. doi:10.1007/s12026-009-8095-8
Schrödinger L The PyMOL Molecular Graphics System. 1.5.0.4 edn
Acknowledgments
This work was supported by NIH grant R01-GM-098977 to CBK and KEG. RSS was supported in part by a Luce Foundation Fellowship and in part by a Thayer Innovation Program Fellowship from the Thayer School of Engineering. The authors would like to thank Thomas Scanlon, Warren Kett, and Deeptak Verma for their insights and support.
Conflict of interest
Karl E. Griswold and Chris Bailey-Kellogg are Dartmouth faculty and co-members of Stealth Biologics, LLC, a Delaware biotechnology company. They acknowledge that there is a potential conflict of interest related to their association with this company, and they hereby affirm that the data presented in this paper is free of any bias. This work has been reviewed and approved as specified in these faculty members’ Dartmouth conflict of interest management plans. The remaining authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salvat, R.S., Parker, A.S., Guilliams, A. et al. Computationally driven deletion of broadly distributed T cell epitopes in a biotherapeutic candidate. Cell. Mol. Life Sci. 71, 4869–4880 (2014). https://doi.org/10.1007/s00018-014-1652-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1652-x