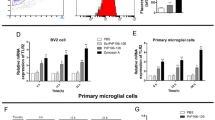
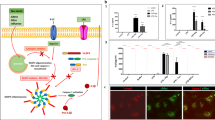
Abstract
Prion diseases are fatal transmissible neurodegenerative diseases, characterized by aggregation of the pathological form of prion protein, spongiform degeneration, and neuronal loss, and activation of astrocytes and microglia. Microglia can clear prion plaques, but on the other hand cause neuronal death via release of neurotoxic species. Elevated expression of the proinflammatory cytokine IL-1β has been observed in brains affected by several prion diseases, and IL-1R-deficiency significantly prolonged the onset of the neurodegeneration in mice. We show that microglial cells stimulated by prion protein (PrP) fibrils induced neuronal toxicity. Microglia and macrophages release IL-1β upon stimulation by PrP fibrils, which depends on the NLRP3 inflammasome. Activation of NLRP3 inflammasome by PrP fibrils requires depletion of intracellular K+, and requires phagocytosis of PrP fibrils and consecutive lysosome destabilization. Among the well-defined molecular forms of PrP, the strongest NLRP3 activation was observed by fibrils, followed by aggregates, while neither native monomeric nor oligomeric PrP were able to activate the NLRP3 inflammasome. Our results together with previous studies on IL-1R-deficient mice suggest the IL-1 signaling pathway as the perspective target for the therapy of prion disease.






Similar content being viewed by others
Abbreviations
- ASC:
-
Apoptosis-associated speck-like protein
- NLRP3:
-
NACHT, LRR, and PYD domains-containing protein 3
- PrP:
-
Prion protein
References
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95(23):13363–13383
Budka H, Aguzzi A, Brown P, Brucher JM, Bugiani O, Gullotta F, Haltia M, Hauw JJ, Ironside JW, Jellinger K et al (1995) Neuropathological diagnostic criteria for Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol 5(4):459–466
Williams AE, Lawson LJ, Perry VH, Fraser H (1994) Characterization of the microglial response in murine scrapie. Neuropathol Appl Neurobiol 20(1):47–55
Betmouni S, Perry VH, Gordon JL (1996) Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience 74(1):1–5
Giese A, Brown DR, Groschup MH, Feldmann C, Haist I, Kretzschmar HA (1998) Role of microglia in neuronal cell death in prion disease. Brain Pathol 8(3):449–457
Suzumura A, Takeuchi H, Zhang G, Kuno R, Mizuno T (2006) Roles of glia-derived cytokines on neuronal degeneration and regeneration. Ann NY Acad Sci 1088:219–229
Baker CA, Manuelidis L (2003) Unique inflammatory RNA profiles of microglia in Creutzfeldt-Jakob disease. Proc Natl Acad Sci USA 100(2):675–679
Tribouillard-Tanvier D, Striebel JF, Peterson KE, Chesebro B (2009) Analysis of protein levels of 24 cytokines in scrapie agent-infected brain and glial cell cultures from mice differing in prion protein expression levels. J Virol 83(21):11244–11253
Schultz J, Schwarz A, Neidhold S, Burwinkel M, Riemer C, Simon D, Kopf M, Otto M, Baier M (2004) Role of interleukin-1 in prion disease-associated astrocyte activation. Am J Pathol 165(2):671–678
Tamguney G, Giles K, Glidden DV, Lessard P, Wille H, Tremblay P, Groth DF, Yehiely F, Korth C, Moore RC, Tatzelt J, Rubinstein E, Boucheix C, Yang X, Stanley P, Lisanti MP, Dwek RA, Rudd PM, Moskovitz J, Epstein CJ, Cruz TD, Kuziel WA, Maeda N, Sap J, Ashe KH, Carlson GA, Tesseur I, Wyss-Coray T, Mucke L, Weisgraber KH, Mahley RW, Cohen FE, Prusiner SB (2008) Genes contributing to prion pathogenesis. J Gen Virol 89(Pt 7):1777–1788
Combrinck MI, Perry VH, Cunningham C (2002) Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience 112(1):7–11
Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH (2005) Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci 25(40):9275–9284
Petrilli V, Dostert C, Muruve DA, Tschopp J (2007) The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol 19(6):615–622
Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440(7081):228–232
Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG (2006) Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126(6):1135–1145
Niemi K, Teirila L, Lappalainen J, Rajamaki K, Baumann MH, Oorni K, Wolff H, Kovanen PT, Matikainen S, Eklund KK (2011) Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol 186(11):6119–6128
Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, Tschopp J, Layland LE, Prazeres da Costa C (2010) Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci USA 107(47):20459–20464
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440(7081):237–241
Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320(5876):674–677
Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9(8):847–856
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464(7293):1357–1361
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9(8):857–865
Hafner-Bratkovic I, Gaspersic J, Smid LM, Bresjanac M, Jerala R (2008) Curcumin binds to the alpha-helical intermediate and to the amyloid form of prion protein—a new mechanism for the inhibition of PrP(Sc) accumulation. J Neurochem 104(6):1553–1564
Hafner-Bratkovic I, Bester R, Pristovsek P, Gaedtke L, Veranic P, Gaspersic J, Mancek-Keber M, Avbelj M, Polymenidou M, Julius C, Aguzzi A, Vorberg I, Jerala R (2011) Globular domain of the prion protein needs to be unlocked by domain swapping to support prion protein conversion. J Biol Chem 286(14):12149–12156
Hafner-Bratkovic I, Gaedtke L, Ondracka A, Veranic P, Vorberg I, Jerala R (2011) Effect of hydrophobic mutations in the H2–H3 subdomain of prion protein on stability and conversion in vitro and in vivo. PLoS ONE 6(9):e24238
Gaspersic J, Hafner-Bratkovic I, Stephan M, Veranic P, Bencina M, Vorberg I, Jerala R (2010) Tetracysteine-tagged prion protein allows discrimination between the native and converted forms. FEBS J 277:2038–2050
Avbelj M, Hafner-Bratkovic I, Jerala R (2011) Introduction of glutamines into the B2–H2 loop promotes prion protein conversion. Biochem Biophys Res Commun 413(4):521–526
Franken KL, Hiemstra HS, van Meijgaarden KE, Subronto Y, den Hartigh J, Ottenhoff TH, Drijfhout JW (2000) Purification of his-tagged proteins by immobilized chelate affinity chromatography: the benefits from the use of organic solvent. Protein Expr Purif 18(1):95–99
Bocharova OV, Breydo L, Parfenov AS, Salnikov VV, Baskakov IV (2005) In vitro conversion of full-length mammalian prion protein produces amyloid form with physical properties of PrP(Sc). J Mol Biol 346(2):645–659
Baskakov IV, Legname G, Baldwin MA, Prusiner SB, Cohen FE (2002) Pathway complexity of prion protein assembly into amyloid. J Biol Chem 277(24):21140–21148
Qi Y, Wang JK, McMillian M, Chikaraishi DM (1997) Characterization of a CNS cell line CAD, in which morphological differentiation is initiated by serum deprivation. J Neurosci 17(4):1217–1225
Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM (2003) Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia 5(6):533–545
Andreoletti O, Berthon P, Levavasseur E, Marc D, Lantier F, Monks E, Elsen JM, Schelcher F (2002) Phenotyping of protein-prion (PrPsc)-accumulating cells in lymphoid and neural tissues of naturally scrapie-affected sheep by double-labeling immunohistochemistry. J Histochem Cytochem 50(10):1357–1370
Novitskaya V, Bocharova OV, Bronstein I, Baskakov IV (2006) Amyloid fibrils of mammalian prion protein are highly toxic to cultured cells and primary neurons. J Biol Chem 281(19):13828–13836
Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11(2):155–161
Bacot SM, Lenz P, Frazier-Jessen MR, Feldman GM (2003) Activation by prion peptide PrP106-126 induces a NF-kappaB-driven proinflammatory response in human monocyte-derived dendritic cells. J Leukoc Biol 74(1):118–125
Spinner DS, Cho IS, Park SY, Kim JI, Meeker HC, Ye X, Lafauci G, Kerr DJ, Flory MJ, Kim BS, Kascsak RB, Wisniewski T, Levis WR, Schuller-Levis GB, Carp RI, Park E, Kascsak RJ (2008) Accelerated prion disease pathogenesis in Toll-like receptor 4 signaling-mutant mice. J Virol 82(21):10701–10708
Chang J, Yang L, Kouadir M, Peng Y, Zhang S, Shi F, Zhou X, Yin X, Zhao D (2012) Antibody-Mediated Inhibition of Integrin alpha5beta1 Blocks Neurotoxic Prion Peptide PrP(106–126)-Induced Activation of BV2 Microglia. J Mol Neurosci 48(1):248–252
Shi F, Yang L, Kouadir M, Yang Y, Wang J, Zhou X, Yin X, Zhao D (2012) The NALP3 inflammasome is involved in neurotoxic prion peptide-induced microglial activation. J Neuroinflamm 9:73
Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14(9):1583–1589
Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB (2004) Synthetic mammalian prions. Science 305(5684):673–676
Wang F, Wang X, Yuan CG, Ma J (2010) Generating a prion with bacterially expressed recombinant prion protein. Science 327:1132–1135
Makarava N, Kovacs GG, Bocharova O, Savtchenko R, Alexeeva I, Budka H, Rohwer RG, Baskakov IV (2010) Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol 119(2):177–187
Kim JI, Cali I, Surewicz K, Kong Q, Raymond GJ, Atarashi R, Race B, Qing L, Gambetti P, Caughey B, Surewicz WK (2010) Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J Biol Chem 285(19):14083–14087
Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B (2005) The most infectious prion protein particles. Nature 437(7056):257–261
Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LA (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol 11(10):897–904
Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O’Hagan DT, Petrilli V, Tschopp J, O’Neill LA, Lavelle EC (2009) Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci USA 106(3):870–875
Prinz M, Heikenwalder M, Schwarz P, Takeda K, Akira S, Aguzzi A (2003) Prion pathogenesis in the absence of Toll-like receptor signalling. EMBO Rep 4(2):195–199
Brown DR, Schmidt B, Kretzschmar HA (1996) Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380(6572):345–347
Brown DR (1999) Prion protein peptide neurotoxicity can be mediated by astrocytes. J Neurochem 73(3):1105–1113
Raeber AJ, Race RE, Brandner S, Priola SA, Sailer A, Bessen RA, Mucke L, Manson J, Aguzzi A, Oldstone MB, Weissmann C, Chesebro B (1997) Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J 16(20):6057–6065
Walsh DT, Betmouni S, Perry VH (2001) Absence of detectable IL-1beta production in murine prion disease: a model of chronic neurodegeneration. J Neuropathol Exp Neurol 60(2):173–182
Tixador P, Herzog L, Reine F, Jaumain E, Chapuis J, Le Dur A, Laude H, Beringue V (2010) The physical relationship between infectivity and prion protein aggregates is strain-dependent. PLoS Pathog 6(4):e1000859
Falsig J, Julius C, Margalith I, Schwarz P, Heppner FL, Aguzzi A (2008) A versatile prion replication assay in organotypic brain slices. Nat Neurosci 11(1):109–117
Beringue V, Demoy M, Lasmezas CI, Gouritin B, Weingarten C, Deslys JP, Andreux JP, Couvreur P, Dormont D (2000) Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J Pathol 190(4):495–502
Tal Y, Souan L, Cohen IR, Meiner Z, Taraboulos A, Mor F (2003) Complete Freund’s adjuvant immunization prolongs survival in experimental prion disease in mice. J Neurosci Res 71(2):286–290
Acknowledgments
We would like to thank Darija Oven and Robert Bremšak for excellent technical assistance, Manuel Ritter and Clarissa Prazeres da Costa (TUM) for valuable discussions, Kurt Wüthrich for providing plasmid encoding mouse PrP ORF and Dona M. Chikaraishi for CAD cell line. This work was funded by Slovenian Research Agency (Z7-2059 to I.H.-B., P4-0176, N5-003, L4-2404 and J1-4170 to R.J.) and by NIH grant AI083713 to K.A.F.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hafner-Bratkovič, I., Benčina, M., Fitzgerald, K.A. et al. NLRP3 inflammasome activation in macrophage cell lines by prion protein fibrils as the source of IL-1β and neuronal toxicity. Cell. Mol. Life Sci. 69, 4215–4228 (2012). https://doi.org/10.1007/s00018-012-1140-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1140-0