Abstract
Objective
To determine if receptor localization into lipid rafts, or the lipid rafts themselves, are important for FcγRIIa effector functions.
Material
Wild-type FcγRIIa or mutant FcγRIIa(C208A) that does not translocate to lipid rafts were transfected into Chinese hamster ovary (CHO) cells which have been shown to be reliable cells for studying FcγR function.
Treatment
Cells were treated with buffer or methyl-β-cyclodextrin (MβCD) to deplete cholesterol and dissolve the structure of lipid rafts.
Methods
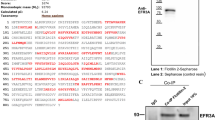
To evaluate lipid raft association, transfected CHO cells were lysed and centrifuged over a sucrose gradient. Fractions were run on SDS-PAGE and blotted for FcγRIIa or sphingolipid GM1 to illustrate the lipid raft fractions. Lateral mobility of GFP-tagged wild-type or mutant FcγRIIa was assessed using fluorescence recovery after photobleaching (FRAP) microscopy. Internalization of IgG-opsonized erythrocytes was assessed by fluorescence microscopy and uptake of heat-aggregated IgG (haIgG) was measured using flow cytometry.
Results
We observed that FcγRIIa(C208A) did not localize into lipid rafts. However, the mutant FcγRIIa retained lateral mobility and effector function similar to wild-type FcγRIIa. However, mutant FcγRIIa function was abolished upon treatment with MβCD.
Conclusions
Lipid rafts provide an essential component required for effector activities independent of receptor localization.



Similar content being viewed by others
References
Vieth JA, Kim MK, Pan XQ, Schreiber AD, Worth RG. Differential requirement of lipid rafts for FcgammaRIIA mediated effector activities. Cell Immunol. 2010;265(2):111–9.
Barnes NC, Powell MS, Trist HM, Gavin AL, Wines BD, Hogarth PM. Raft localisation of FcgammaRIIa and efficient signaling are dependent on palmitoylation of cysteine 208. Immunol Lett. 2006;104(1–2):118–23.
Bournazos S, Hart SP, Chamberlain LH, Glennie MJ, Dransfield I. Association of FcgammaRIIa (CD32a) with lipid rafts regulates ligand binding activity. J Immunol. 2009;182(12):8026–36.
Garcia-Garcia E, Brown EJ, Rosales C. Transmembrane mutations to FcgammaRIIA alter its association with lipid rafts: implications for receptor signaling. J Immunol. 2007;178(5):3048–58.
Mansfield PJ, Hinkovska-Galcheva V, Borofsky MS, Shayman JA, Boxer LA. Phagocytic signaling molecules in lipid rafts of COS-1 cells transfected with FcgammaRIIA. Biochem Biophys Res Commun. 2005;331(1):132–8.
Katsumata O, Hara-Yokoyama M, Sautes-Fridman C, Nagatsuka Y, Katada T, Hirabayashi Y, et al. Association of FcgammaRII with low-density detergent-resistant membranes is important for cross-linking-dependent initiation of the tyrosine phosphorylation pathway and superoxide generation. J Immunol. 2001;167(10):5814–23.
Barabe F, Rollet-Labelle E, Gilbert C, Fernandes MJ, Naccache SN, Naccache PH. Early events in the activation of Fc gamma RIIA in human neutrophils: stimulated insolubilization, translocation to detergent-resistant domains, and degradation of Fc gamma RIIA. J Immunol. 2002;168(8):4042–9.
Kono H, Suzuki T, Yamamoto K, Okada M, Yamamoto T, Honda Z. Spatial raft coalescence represents an initial step in Fc gamma R signaling. J Immunol. 2002;169(1):193–203.
Cuschieri J. Implications of lipid raft disintegration: enhanced anti-inflammatory macrophage phenotype. Surgery. 2004;136(2):169–75.
Hoessli DC, Ilangumaran S, Soltermann A, Robinson PJ, Borisch B, Nasir Ud D. Signaling through sphingolipid microdomains of the plasma membrane: the concept of signaling platform. Glycoconj J. 2000;17(3–4):191–7.
Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768(6):1311–24.
Flaswinkel H, Barner M, Reth M. The tyrosine activation motif as a target of protein tyrosine kinases and SH2 domains. Semin Immunol. 1995;7(1):21–7.
Ghazizadeh S, Bolen JB, Fleit HB. Tyrosine phosphorylation and association of Syk with Fc gamma RII in monocytic THP-1 cells. Biochem J. 1995;305(Pt 2):669–74.
Joshi T, Butchar JP, Tridandapani S. Fcgamma receptor signaling in phagocytes. Int J Hematol. 2006;84(3):210–6.
Greenberg S. Modular components of phagocytosis. J Leukoc Biol. 1999;66(5):712–7.
Hunter S, Kamoun M, Schreiber AD. Transfection of an Fc gamma receptor cDNA induces T cells to become phagocytic. Proc Natl Acad Sci USA. 1994;91(21):10232–6.
Worth RG, Kim MK, Kindzelskii AL, Petty HR, Schreiber AD. Signal sequence within Fc{gamma}RIIA controls calcium wave propagation patterns: Apparent role in phagolysosome fusion. Proc Natl Acad Sci USA. 2003;100:4533–8.
Booth JW, Kim MK, Jankowski A, Schreiber AD, Grinstein S. Contrasting requirements for ubiquitylation during Fc receptor-mediated endocytosis and phagocytosis. EMBO J. 2002;21(3):251–8.
Daniels AB, Worth RG, Dickstein RJ, Dickstein JS, Kim-Han TH, Kim MK, et al. Analysis of FcgammaRIIA cytoplasmic tail requirements in signaling for serotonin secretion: evidence for an ITAM-dependent, PI3 K-dependent pathway. Scand J Immunol. 2010;71(4):232–9.
Worth RG, Mayo-Bond L, Kim MK, van de Winkel JG, Todd RF 3rd, Petty HR, et al. The cytoplasmic domain of FcgammaRIIA (CD32) participates in phagolysosome formation. Blood. 2001;98(12):3429–34.
Worth RG, Mayo-Bond L, van de Winkel JG, Todd RF 3rd, Petty HR. CR3 (alphaM beta2; CD11b/CD18) restores IgG-dependent phagocytosis in transfectants expressing a phagocytosis-defective Fc gammaRIIA (CD32) tail-minus mutant. J Immunol. 1996;157(12):5660–5.
Worth RG, Chien CD, Chien P, Reilly MP, McKenzie SE, Schreiber AD. Platelet FcgammaRIIA binds and internalizes IgG-containing complexes. Exp Hematol. 2006;34(11):1490–5.
Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138(1):141–3.
Hinkovska-Galcheva V, Boxer LA, Kindzelskii A, Hiraoka M, Abe A, Goparju S, et al. Ceramide 1-phosphate, a mediator of phagocytosis. J Biol Chem. 2005;280(28):26612–21.
Kwiatkowska K, Frey J, Sobota A. Phosphorylation of FcgammaRIIA is required for the receptor-induced actin rearrangement and capping: the role of membrane rafts. J Cell Sci. 2003;116(Pt 3):537–50.
Korzeniowski M, Kwiatkowska K, Sobota A. Insights into the association of FcgammaRII and TCR with detergent-resistant membrane domains: isolation of the domains in detergent-free density gradients facilitates membrane fragment reconstitution. Biochemistry. 2003;42(18):5358–67.
Abdel Shakor AB, Kwiatkowska K, Sobota A. Cell surface ceramide generation precedes and controls FcgammaRII clustering and phosphorylation in rafts. J Biol Chem. 2004;279(35):36778–87.
Strzelecka-Kiliszek A, Korzeniowski M, Kwiatkowska K, Mrozinska K, Sobota A. Activated FcgammaRII and signalling molecules revealed in rafts by ultra-structural observations of plasma-membrane sheets. Mol Membr Biol. 2004;21(2):101–8.
Tse SM, Furuya W, Gold E, Schreiber AD, Sandvig K, Inman RD, et al. Differential role of actin, clathrin, and dynamin in Fc gamma receptor-mediated endocytosis and phagocytosis. J Biol Chem. 2003;278(5):3331–8.
Mero P, Zhang CY, Huang ZY, Kim MK, Schreiber AD, Grinstein S, et al. Phosphorylation-independent ubiquitylation and endocytosis of Fc gammaRIIA. J Biol Chem. 2006;281(44):33242–9.
Katagiri YU, Kiyokawa N, Fujimoto J. A role for lipid rafts in immune cell signaling. Microbiol Immunol. 2001;45(1):1–8.
Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319(5860):210–3.
Yeung T, Terebiznik M, Yu L, Silvius J, Abidi WM, Philips M, et al. Receptor activation alters inner surface potential during phagocytosis. Science. 2006;313(5785):347–51.
Acknowledgments
This work was supported by an Arthritis Foundation Investigator Award (to R.G.W.) and National Institutes of Health grant HL-28207 (to A.D.S.). The authors would like to thank Dr. Andrea Kalinoski in the University of Toledo Advanced Microscopy and Imaging Center (AMIC) and the Flow Cytometry Core facility for technical assistance and use of the equipment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Liwu Li.
Rights and permissions
About this article
Cite this article
Vieth, J.A., Kim, Mk., Glaser, D. et al. FcγRIIa requires lipid rafts, but not co-localization into rafts, for effector function. Inflamm. Res. 62, 37–43 (2013). https://doi.org/10.1007/s00011-012-0548-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-012-0548-1