Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation that is not fully reversible; this airflow limitation is both progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gasses. COPD is undoubtedly an umbrella term, and it seems unlikely that all patients with COPD have the same underlying disease processes; thus, there is a need for differential treatment of different subgroups. A potential solution is to find modifiable biomarkers that can assist in drug development and distinguish subgroups of COPD. With the exception of lung function tests, there are currently no well-validated biomarkers or surrogate endpoints that can be used to establish the efficacy of a drug for COPD. This article discusses biomarkers of inflammation (fibrinogen, C-reactive protein, pulmonary and activation-regulated chemokine/CC-chemokine ligand-18, serum surfactant protein D, interleukin (IL)-6, IL-8 and tumor necrosis factor α, complement factor C5a), angiogenesis factors as a part of the pathogenetic aspect in this disease (vascular endothelial growth factor, angiogenin, and IL-8), and matrix degradation biomarkers. Troponin and natriuretic peptides are presented as biomarkers of cardiac involvement in the light of COPD comorbidities. Trials based on research on known clinical variables such as FEV1, BODE, and 6MWT in combination with biomarkers from lung and blood specimens will probably clarify part of the prognosis and natural history of the disease. This will also represent an additional step in COPD phenotyping and new treatment possibilities.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease, the fourth-leading cause of death worldwide, is characterized by airflow limitation that is not fully reversible; this airflow limitation is both progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gasses (Pauwels et al. 2001; World Health Organization 2008). Forced expiratory volume in one second (FEV1) is the traditional marker used to define the progression of COPD and the strongest spirometric predictor of mortality in COPD patients (Thomason and Strachan 2000). Factors that affect the decline in pulmonary function viewed through FEV1 are cigarette smoking and exacerbation frequency (Anthonisen et al. 1994; Donaldson et al. 2002). A number of risk factors have been associated with frequent acute exacerbations of COPD: hypercapnia, previous hospital admissions, current smoking, impaired health status, pulmonary hypertension, hypoxemia, low body mass index, and low FEV1 (Garcia-Aymerich et al. 2001; Kessler et al. 1999; Oostenbrink and Rutten-van Molken 2004; Soler et al. 1998). In addition to pulmonary functional defect, COPD is also associated with significant systemic effects outside the lungs, such as malnutrition, pulmonary hypertension, and peripheral muscle weakness (Agusti 2005; Mahler and Criner 2007). COPD is thus associated with both airway and systemic inflammation but there is a lack of information on how this inflammation changes over time (Donaldson et al. 2005) and during disease exacerbation. COPD is undoubtedly an umbrella term, and it seems unlikely that all patients with COPD have the same underlying disease processes; thus, there is a need for differential treatment of different subgroups. A potential solution is to find modifiable biomarkers that can assist in drug development and distinguish subgroups of COPD (Sin and Vestbo 2009). Moreover, COPD is frequently accompanied by comorbidities such as heart disease. It would be useful to identify a biomarker that is disease specific, thus enable differentiating the progression rate of each of the comorbid conditions.
This review highlights the role of COPD biomarkers, which is believed to be involved in the pathogenetic process of COPD at the level of inflammation, angiogenesis, matrix degradation, and cardiac involvement. With the growing awareness of COPD as a systemic disease and a shift of biomarker research to blood specimens, this review primarily discusses blood biomarkers. They are discussed in the light of clinical outcome in these patients. FEV1, the BODE index, and the 6-minute walk test (6MWT) are used as measures of functional impairment or outcome markers. The article also highlights study approaches such as the value of adding a panel of biomarkers to clinical variables known to predict mortality in COPD and a biomarker “network”.
Biomarkers as a Tool to Evaluate Pathogenic Processes in COPD
The National Institutes of Health define biomarkers as a “characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (De Gruttola et al. 2001). Bucher et al. (1999) proposed two essential criteria for assessing the validity of biomarkers. First, is there a strong, independent, consistent association between the surrogate end point and the clinical end point? Second, is there evidence from randomized controlled trials that improvement in the surrogate end point with a drug consistently leads to improvement in the clinical outcome? Sin and Vestbo (2009) propose that in COPD these criteria can be extended to five essential questions, which are presented in Table 1.
The US Food and Drug Administration states that, currently, “with the exception of lung function tests, there are no well-validated biomarkers or surrogate endpoints that can be used to establish efficacy of a drug for COPD” (US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) 2007). In theory, biomarkers can be obtained from any source. In COPD, the most logical source is the lung. Potential biomarker sources include bronchial biopsies and bronchoalveolar lavage, which are highly invasive and thus difficult to repeat. Exhaled gasses and breath condensate are considerably less invasive, but results are highly variable (Celli 2010). Breath condensate may be improved using standardized methods and by performing sequential sampling and averaging these results (Sapey et al. 2008; Vignola et al. 2002). With the growing awareness of COPD as a systemic disease, there has been a shift to biomarker research in blood specimens (Malhotra et al. 2006).
However, according to the definition (De Gruttola et al. 2001), data from functional assessment of COPD patients can also be used as a biomarker. FEV1, the BODE index, and the 6MWT are used in functional assessment of these patients, for example. At the moment they represent clinical functional biomarkers that are used in everyday clinical practice.
In addition to functional biomarkers for COPD, which are used in everyday clinical practice, various biochemical and immunological biomarkers might be introduced according to their pathophysiological processes, including inflammation, cardiac involvement, matrix destruction, and angiogenesis. Some of these are present in clinical use, whereas others are novel and emerging. These data are summarized in Table 2.
According to Cazzola et al. (2008), the term “biomarker” refers to the measurement of any molecule or material (e.g., cells, tissue, etc.) that reflects the disease process. It must be stressed that FEV1, the BODE index, and the 6MWT are important outcome markers and according to Cazzola et al. (2008) do not represent a pure classical biomarker. They are included in this article as biomarkers, but it must be stressed that they primarily have the role of important outcome markers.
Functional Biomarkers for COPD
FEV1
FEV1 is relatively easy to obtain and is used to track health outcomes in COPD (Sin and Vestbo 2009). However, for short-term drug trials it is not an ideal surrogate because it does not provide information on the underlying pathologic process (Sutherland and Cherniack 2004), cannot separate the various phenotypes of COPD (Calverley et al. 2007), is not specific for COPD because it is also altered in other comorbidities (De Gruttola et al. 2001), and it is relatively unresponsive to known therapies that prolong survival (supplemental oxygen) (Bucher et al. 1999). FEV1 is thus a good marker for prognosis of COPD patients, but it is too inexact and suboptimal in assessing the pathologic processes in COPD. In terms of health outcomes for the general population, low pre-bronchodilator FEV1 has been found to be a predictor of all-cause mortality and cardiovascular mortality (Cazzola et al. 2008; Neas and Schwartz 1998). In COPD patients, post-bronchodilator FEV1 is poorly correlated with patient-centered outcomes such as dyspnea, exercise performance, and HRQol at baseline or after pharmacological interventions (Hajiro et al. 1998).
An observational study of patients with COPD found that the rate of decline in FEV1 over a 3-year period is highly variable (Vestbo et al. 2011). Although COPD is considered to be a progressive disease, only 38 % of patients had an estimated rate of decline in FEV1 of more than 40 ml per year. Current smoking was most strongly associated with the rate of decline in FEV1. The mean rate of decline in FEV1 was 21 ± 4 ml per year greater in current smokers than in current nonsmokers. In the same observational study they found that patients with emphysema and patients with bronchodilator reversibility both had excessive loss of FEV1. In emphysema the rate of decline was 13 ± 4 ml greater than in those without emphysema and in patients with bronchodilator reversibility it was 17 ± 4 ml per year greater than in those without it. At the same time they studied a number of biomarkers [fibrinogen, interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, C-reactive protein (CRP), Clara cell protein 16 and surfactant protein D]. They found that only Clara cell protein was associated with the rate of decline in FEV1. But this association is weak and whether it is biologically meaningful has yet to be determined.
Measurement of FEV1 is possible during exacerbations of COPD but it does not seem useful for early detection of exacerbations (Seemungal et al. 2000). The mean number of exacerbations is generally related to the severity of the baseline disease. If unreported exacerbations are included, severe patients (GOLD III) have a mean of 3.43 exacerbations and GOLD II patients have a mean of 2.68 per year (Cazzola et al. 2008; Donaldson et al. 2003).
Functional Assessment with the 6MWT and BODE Index
The risk of death in patients with COPD is often graded with the use of the FEV1 (Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011). However, other risk factors such as the presence of hypoxemia or hypercapnia (1983; 1980), a short distance walked in a fixed time (Gerardi et al. 1996), and a low body mass index (the weight in kilograms divided by the square of the height in meters) (Schols et al. 1998) are also associated with an increased risk of death. Thus, although FEV1 is important to obtain and essential in the disease staging in any patient with COPD, other variables provide useful information that can improve the comprehensibility of the evaluation of patients with COPD (Celli et al. 2004). FEV1 does not adequately reflect all the systemic manifestations of the disease. It has, for example, a weak correlation with the degree of dyspnea (Celli et al. 2004; Mahler et al. 1984). Dyspnea is one of the most disabling symptoms of COPD; the degree of dyspnea provides information regarding the patient’s perception of illness and can be measured. The modified Medical Research Council (MMRC) dyspnea scale is simple to administer and correlates with other dyspnea scales (Celli et al. 2004; Mahler et al. 1984).
The 6MWT is important in assessing functional exercise capacity in patients with chronic respiratory disease. It is reliable, inexpensive, easy to perform, safe, and it has been standardized (ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories 2002). The distance walked in 6 min has been accepted as a good outcome measure after pulmonary rehabilitation (1999). The BODE index combines the four variables mentioned (FEV1, dyspnea, body mass index, and 6MWT) by the means of a simple scale (Celli et al. 2004). It is a multidimensional index and it is a better predictor of the risk of death from any cause and from respiratory causes than is the FEV1 alone. The BODE index is useful because it includes one domain that quantifies the degree of pulmonary impairment (FEV1), one that includes the patient’s perception of symptoms (the MMRC dyspnea scale), and two independent domains (the distance walked in 6 min and the body mass index) that express the systemic consequences of COPD (Celli et al. 2004). A recent study by Celli hypothesized that the addition of a panel of biomarkers to clinical variables known to predict mortality in COPD such as age, FEV1, BODE, or hospitalizations due to exacerbations of the disease will improve accuracy in predicting the risk of death in patients with COPD. Celli concluded that the addition of white blood cell counts and systemic levels of IL-6, CRP, IL-8, fibrinogen, Clara cell secretory protein-16, and surfactant protein-D (SP-D) significantly improve the ability of clinical variables to predict mortality in patients with COPD (Celli et al. 2012).
Biomarkers of Inflammation
Plasma Fibrinogen
Fibrinogen is an acute phase reactant and a blood clotting factor that is synthesized by hepatocytes and released in large amounts into the circulation primarily in response to IL-6 stimulation (Castell et al. 1989; Gabay and Kushner 1999). It is therefore possible that fibrinogen could be used as a noninvasive measurement of ongoing airway inflammation and lung tissue destruction (Dahl et al. 2001). Dahl et al. have proposed that pulmonary inflammation in COPD is associated with increased levels of acute phase reactants in plasma, and that these reactants could potentially be used to predict risk of future COPD events. To test whether the acute phase reactant fibrinogen predicts future COPD, they determined lung function and relative risk of COPD hospitalizations in individuals with increased levels of plasma fibrinogen. They found that individuals with baseline plasma fibrinogen of more than 3.3 g/l versus less than 2.7 g/l had reduced lung function, increased cumulative incidence of COPD hospitalizations, and an increased risk of 1.7 (1.1–2.6) for COPD hospitalization during 6 years of follow-up (Dahl et al. 2001; Dahl and Nordestgaard 2009).
A later study confirmed plasma fibrinogen as a marker of COPD prognosis. Gan et al. (2004) estimated a 0.37 g/l difference in plasma fibrinogen between COPD patients and controls. A longitudinal study by Donaldson et al. (2005) with a well-characterized patient cohort with moderate to severe COPD examined longitudinal changes in airway and systemic inflammatory markers and prospectively related levels of these markers to FEV1 decline. The conclusion was that patients with high indexes that had stable plasma fibrinogen, sputum neutrophils, sputum eosinophils, and a sputum IL-8 had a more rapid decline in FEV1. The same study was unable to show a relationship between stable sputum IL-6 and plasma fibrinogen, suggesting that there is no direct link between systemic and airway inflammation. It suggests that the origin of the systemic response is not caused solely by release of airway inflammatory mediators into the systemic circulation, and further research is required in this area. While exploring the relationship between COPD exacerbation frequency and airway and systemic inflammation they found that frequent exacerbators (exacerbation frequency of >2.52 per year) were more likely to show a faster rise in plasma fibrinogen and sputum IL-6 over time than patients with a history of infrequent exacerbations. The authors suggest that the mechanism by which these acute events contribute to the chronic deterioration in FEV1 decline is by causing a faster rate of increase in airway inflammation. The elevated systemic inflammatory response in frequent exacerbators suggests that this group may be particularly prone to muscle weakness with increasing disease severity and thus may be an important group to target with pulmonary rehabilitation (Bhowmik et al. 2000). Moreover, some very recently published data showed that a panel of selected biomarkers that included fibrinogen and CRP were not only elevated in nonsurvivors compared with survivors, but were also associated with mortality over 3 years (Celli et al. 2012). Epidemiological studies have shown that more than 40 % of patients with COPD have concomitant cardiovascular disease, where chronic heart failure (CHF) is among the most frequent ones (Chatila et al. 2008). Patients with CHF and cachexia also have an increased hepatic fibrinogen synthesis (Witte et al. 2008) and fibrinogen has been identified as an independent prognostic factor for cardiovascular morbidity (Danesh et al. 2005). Therefore, the increased level of fibrinogen in patient with COPD has to be interpreted carefully and in the light of patient’s comorbidities.
C-Reactive Protein
The most commonly used acute phase reactant in clinical situations, CRP, binds bacteria (Mold et al. 2002; Weiser et al. 1998), oxidized lipids (Chang et al. 2002; van Tits et al. 2005), and apoptotic cells (Gershov et al. 2000) and facilitates their clearance via the innate immune system. There is evidence suggesting that increased serum CRP levels also associate with lung inflammation in stable COPD (de Torres et al. 2006; Kony et al. 2004; Pinto-Plata et al. 2006). In a cohort study of Dahl they tested the hypothesis that increased concentrations of serum CRP predicts increased risk of hospitalization and death from COPD in individuals with airway obstruction. They found that individuals with serum CRP of more than 3 mg/l versus less than 3 mg/l at baseline had increased cumulative incidence of COPD hospitalization and death, an increased hazard ratio of 1.4 (1.0–2.0) for COPD hospitalization and an increased hazard ratio of 2.2 (1.2–3.9) for COPD death. Absolute risks for COPD hospitalization and death increased with baseline serum CRP. The data suggest an important role for CRP, or its main regulatory cytokine IL-6, in COPD progression and development (Dahl et al. 2007; Dahl and Nordestgaard 2009). Supporting a role for IL-6 in COPD pathogenesis, IL-6 may stimulate inflammatory cell recruitment in the lung and leads to emphysema-like airspace enlargement and respiratory muscle wasting in animal models of lung disease (DiCosmo et al. 1994; Hierholzer et al. 1998; Johnston et al. 2005). In the future, CRP levels could be used clinically to assess prognosis in patients with airway obstruction.
There are some reasons for caution in linking CRP and COPD outcome (Donaldson 2007). First, CRP increases in response to a number of infectious and inflammatory conditions and therefore it is not specific to COPD. Second, it has been estimated that 40–50 % of the variation in serum CRP levels is due to inherited characteristics (Wouters 2006). Third, although inhaled corticosteroids can dramatically lower CRP levels by up to 50 % (Sin et al. 2004), the evidence that they reduce COPD mortality is much less clear-cut and any benefits may be limited to reducing cardiovascular mortality (Macie et al. 2006).
In patients with mild to moderate COPD, Man et al. (2006) have recently reported a positive trend between quintiles of CRP and increased cardiovascular, cerebrovascular, and cancer deaths, but not deaths from respiratory disease. These results fit with a number of studies that have shown that an elevated CRP will predict mortality from cardiovascular causes in patients with other diseases, such as diabetes or renal disease (Racki et al. 2006).
Pulmonary and Activation-Regulated Chemokine/CC-Chemokine Ligand-18
This is a 7-kD protein that is expressed by monocytes/macrophages and dendritic cells and is predominantly excreted in the lungs (Gunther et al. 2005). In a small study of patients with mild to moderate COPD, serum pulmonary and activation-regulated chemokine/CC-chemokine ligand-18 (PARC/CCL-18) was significantly associated with reduced FEV1, increased risk of exacerbations, and an increased BODE index (Pinto-Plata et al. 2007). There is also recent evidence that elevated levels of PARC/CCL-18 are associated with increased risk of cardiovascular hospitalizations or mortality in the lung health study (LHS) cohort and with total mortality in the ECLIPSE cohort (Sin et al. 2011). Circulating levels of PARC/CCL-18 have been found to be associated with acute exacerbations (Hurst et al. 2006). It should also be noted that although the expression of this chemokine occurs mostly in the lungs, other organs including the prostate, bone marrow, blood vessels, and liver also express this protein. Its levels also rise during ischemic myocardial events and these levels predict the future risk of cardiovascular morbidity and mortality (Kraaijeveld et al. 2007). This may explain why researchers have observed a significant relationship in the LHS between serum PARC/CCL-18 concentrations and the risk of cardiovascular hospitalization and mortality (Sin et al. 2011). PARC/CCL-18, with the addition of other inflammatory biomarkers such as white blood count (WBC), CRP, IL-8, fibrinogen, and SP-D, significantly improves the ability of clinical variables to predict mortality in patients with COPD (Celli et al. 2012).
Serum SP-D
SP-D is a large hydrophilic protein that is one of the collagen-containing C-type lectins. It is found in the endoplasmic reticulum of type II pneumocytes and the secretory granules of Clara cells. It contributes to surfactant homeostasis and pulmonary immunity (Kishore et al. 2006; Mori et al. 2002). The ECLIPSE cohort was used to evaluate serum SP-D as a biomarker for COPD. The median serum SP-D levels were significantly higher in current and former smokers with COPD than in those without airflow obstruction. Moreover, the risk of exacerbation increased with increasing baseline serum SP-D concentrations. There was no significant increase in serum levels with increasingly severe disease as assessed by GOLD score. The largest difference in serum SP-D levels occurred between nonsmokers and current/former smokers. The authors concluded that serum SP-D is a powerful biomarker for smoking and that it reflects intrapulmonary inflammation, which would explain the higher levels in smokers with and without COPD. Moreover, there was a rapid and marked fall in serum SP-D levels while individuals with COPD were receiving oral corticosteroids (Lomas et al. 2009). This reflects the role of SP-D in lung inflammation and is a possible target of anti-inflammatory therapy for COPD.
IL-6, IL-8, and TNF-α
IL-6 modulates the number and/or activity of important inflammatory cells (Chomarat et al. 2000; Suwa et al. 2001). IL-6 is synthesized by airway epithelium, macrophages, and several other cells at sites of inflammation in response to environmental stress (Martin et al. 1997), such as smoking. It is involved as a primary cytokine regulating the expression of fibrinogen (Castell et al. 1989) and has a regulatory role in the synthesis of CRP.
IL-8 is a chemokine that is produced by the bronchial epithelium and macrophages and is a potent neutrophil activator and chemoattractant, and TNF-α is a cytokine that is produced by many cells (e.g., macrophages, T cells, mast cells, and epithelial cells). Elevated circulating levels of CRP, IL-6, IL-8, and TNF-α were found in patients with COPD (Agusti 2007; Gan et al. 2004; Fabbri and Rabe 2007). Several authors have therefore suggested systemic inflammation as a potential factor in the development of complications and comorbidities, including cachexia, in these patients (Agusti et al. 2003; Barnes and Celli 2009; Nussbaumer-Ochsner and Rabe 2011).
A recent study hypothesized that the persistence of systemic inflammation in COPD constitutes a novel COPD phenotype (Agusti et al. 2012). It examined the prevalence, temporal stability, and network pattern of the six inflammatory biomarkers most often studied in COPD (WBC count, CRP, IL-6, IL-8, fibrinogen, and TNF-α) and their relationship with clinical characteristics and outcomes at the 3-year follow-up. This study is based on an ECLIPSE cohort and provides three relevant and novel observations. First, it characterizes the systemic inflammatory network pattern in patients with COPD and distinguishes it from that of smokers with normal pulmonary function and nonsmokers. Second, it shows that systemic inflammation is not a constant feature in all COPD patients because about one-third did not have an abnormal biomarker baseline level and about the same proportion remained “not inflamed” after 1 year of follow-up. Finally, there was a subgroup of patients with COPD with persistently elevated levels of biomarkers and significantly increased all-cause mortality and exacerbation frequency.
The idea of a study approach to the biomarker “network” in COPD is particularly interesting. Eagan et al. (2012) very recently prospectively studied the relationship between the systemic inflammatory biomarkers CRP, TNF-α, IL-β, and IL-6 and loss of lean body mass over a 3-year period. Multivariately sustained high levels of TNF-α, but not CRP, IL-β, or IL-6, significantly predicted loss of lean body mass; however, this was true only in patients with established cachexia at entry. The study therefore does not support the hypothesis that systemic inflammation is the cause of accelerated loss of lean body mass in COPD patients, but suggests a role for TNF-α in already cachectic COPD patients (Eagan et al. 2012).
On the other hand, TNF-α production was associated with muscle loss and weakness in COPD (Di Francia et al. 1994; Schols et al. 1998). Based on facts known about the role of TNF-α at the level of inflammation and systemic effects, TNF-α was considered a possible target for new drugs in COPD. Anti-TNF-α, infliximab, has been studied (phase II, double blind, multicenter, placebo controlled) to evaluate the safety and efficacy of infliximab (Rennard et al. 2007). It failed to demonstrate improvement in the chronic respiratory questionnaire score and other secondary clinical outcomes after 24 weeks of treatment. Recently the study sought to evaluate the systemic inflammatory profile associated with COPD and to access the impact of tumor necrosis factor neutralization on systemic inflammation (Loza et al. 2012). It was concluded that treatment with infliximab (anti-TNF-α) did not significantly impact the expression of COPD-associated inflammatory markers in serum.
Complement Factor C5a
C5a is an activated component of the complement system that in addition to playing an important role in the defense system contributes to the amplification of inflammation if activated in excess or inappropriately controlled. C5a is a very potent molecule, and it has been shown to have the potential to promote innate and adaptive inflammatory responses in many tissues, including lung tissue. In addition to its chemotactic role, C5a is involved in enhancing production of various cytokines, enhances vascular permeability, regulates vasodilatation, and influences the adaptive immune system by stimulating Th1 response (Köhl 2001).
Generation of C5a in the airways may occur through various mechanisms; C5a may be cleaved by proteases released from mast cells and pulmonary macrophages, or it may be cleaved through the activation of various complement pathways (Fukuoka et al. 2008; Wills-Karp 2007).
In vitro and in vivo experiments suggest that C5a may be involved in disorders including adult respiratory distress syndrome, systemic lupus erythematosu, rheumatoid arthritis, septic syndrome, ischemia reperfusion injury, and psoriasis. Based on our previous studies, C5a could also be involved in the pathogenesis of COPD (Marc et al. 2004). In this study in patients with stable COPD we found elevated in vivo levels of C5a in induced sputum compared to healthy non-smoking subjects. In our later work (Marc et al. 2010), we studied the airway and systemic levels of C5a and C3a in patients with COPD during the stable phase and during acute exacerbation of the disease. For this purpose we analyzed induced sputum and plasma samples of 28 patients with COPD. In 13 of these patients, we also obtained samples during acute exacerbation of the disease. Compared to healthy controls, we found higher C5a concentrations in the airways of patients with stable COPD that significantly negatively correlated with FEV1 and TLCO (lung diffusion capacity). We also documented significantly higher systemic C5a levels in patients with stable COPD. Most importantly, local C5a concentrations significantly increased during acute exacerbation of COPD. This increase was not associated with the plasma C5a values. This evidence suggests independent local C5a production during exacerbation. Our findings support the importance of C5a in the pathogenesis of COPD. They also support continued investigation into C5a for the development of new treatments that might affect COPD exacerbation.
Biomarkers of Cardiac Involvement
Natriuretic Peptides and Troponin T
Atrial natriuretic peptide (ANP) is a peripheral and pulmonary artery vasodilator that is released from myocardial cells in the atria, and in some cases in the ventricles, as a response to increased cardiac wall stress and volume expansion (Adnot et al. 1989; Espiner et al. 1995). The discovery of ANP in 1984 was followed by B-type natriuretic peptide (BNP) later (Lainscak et al. 2009). Nonetheless, only BNP and its precursor, N-terminal pro-B-type natriuretic peptide (NT-proBNP), have been accepted into clinical heart failure guidelines (Swedberg et al. 2005). They both are established markers of left ventricular dysfunction and are associated with increased mortality in acute and stable heart disease (de Lemos et al. 2001; Kragelund et al. 2005). Cardiac troponins are specific markers of myocardial necrosis and are used to diagnose myocardial infarction (Alpert et al. 2000). Elevated troponin levels may also occur in pulmonary thromboembolism, congestive heart failure, tachyarrhythmias, myocarditis, pericarditis, sepsis, and stroke (Mahajan et al. 2006). Troponin elevations in these conditions probably reflect general myocardial injury rather than coronary arterial occlusion. Some data indicate that cardiac troponins are frequently elevated in COPD exacerbations and appear to be associated with the severity of the exacerbation (Harvey and Hancox 2004).
Epidemiological studies have shown that more than 40 % of patients with COPD have concomitant cardiovascular disease, of which CHF is among most frequent (Chatila et al. 2008). The degree to which cardiac disease contributes to mortality during exacerbation of COPD is unknown. Recently some investigators focused on this topic with an investigation of NT-proBNP in patients with acute exacerbation of COPD without coronary ischemia. These recent interesting data published by Chang et al. (2011) showed that elevated NT-proBNP and troponin T were strongly associated with increased early mortality in patients admitted to the hospital with exacerbation of COPD. Moreover, patients with abnormalities in both NT-proBNP and troponin T had 15-fold higher mortality at 30 days than patients with normal values for both markers (Chang et al. 2011). It is unclear whether elevated levels of NT-proBNP were due to left or right ventricular dysfunction since BNP and NT-proBNP might be elevated in patients with the diseased pulmonary vessels as in pulmonary arterial hypertension and pulmonary thromboembolism (Nagaya et al. 2000; Tulevski et al. 2001). It is known that the pathophysiology during the exacerbation of severe COPD includes hypoxemia, hypoxic vasoconstriction, aggravation of pulmonary hypertension, and right ventricular dysfunction (Chaouat et al. 2008) and, in combination with tachycardia, it thus might represent the source of elevated NT-proBNP and troponin T. The authors concluded that although both biomarkers predicted mortality independently of other prognostic indicators, it remains unclear whether cardiac involvement is a direct cause of mortality or whether these biomarkers just reflect the severity of exacerbation. This theory is absolutely watertight, but the gap between clinical practice and theoretical points is sometimes very wide. What do we actually do in clinical practice in patients with severe exacerbation of COPD? If we decide to measure troponin T and NT-proBNP and we find them elevated we usually do everything we can to rule out conditions such as pulmonary embolism and serious acute heart disease, searching for heart failure or an acute coronary event. Only after ruling out such serious events, which might mimic acute exacerbation of COPD, can changes in biomarkers be attributed to acute exacerbation of COPD. Therefore, elevated troponin T and elevated NT-proBNP in acute settings (without pulmonary embolism or acute coronary event or obvious heart failure) might help clinicians assess prognosis, but at the moment there is insufficient data for making recommendations about their influence in changing the treatment of acute exacerbation of COPD. In a very recent study, Marcun et al. (2012) reported that discharge troponin T predicted hospitalizations (hazard ratio: 2.89, 95 % confidence interval: 1.13–7.36) and that admission NT-proBNP predicted mortality.
The mid-regional pro-atrial natriuretic peptide (MR-proANP) has a much longer half life than mature ANP and has been suggested as a more reliable analyte for measurement. In a study of serum levels of MR-proANP in patients with COPD, Bernasconi et al. (2011) reported three major findings. First, MR-proANP was elevated in patients hospitalized for exacerbation of COPD compared with their levels during recovery and the stable phase of the disease. Second, MR-proANP levels at exacerbation were increased in long-term nonsurvivors compared with survivors. Third, MR-proANP and PaCO2 were independent predictors of 2-year survival in patients with COPD. These indicate that MR-proANP might therefore have had a prognostic value in this group of patients.
Biomarkers of Angiogenesis
The Role of Angiogenic Factors, Vascular Endothelial Growth Factor, Angiogenin, and IL-8
Angiogenesis is a prominent feature of the structural tissue remodeling that occurs in the chronic airway diseases including COPD, and local production of vascular endothelial growth factor (VEGF) has been implicated as a major driver of angiogenesis in the airway component of COPD. VEGF production is up-regulated by several factors, including hypoxia, acidosis, and growth factors (Kanazawa et al. 2003; Kasahara et al. 2000; Walters et al. 2008). VEGF is one of the most potent mediators of vascular regulation, angiogenesis, and vascular permeability (Ferrara 2000; Papaioannou et al. 2006).
VEGF and its receptors have been detected in alveolar type II cells, airway epithelial cells, macrophages, and neutrophils, as well as airway smooth muscle cells (Kaner and Crystal 2001; Kazi et al. 2004; Mura et al. 2004). Studies on VEGF in the pathogenesis of two different pathological entities of COPD (emphysema and chronic bronchitis) suggest a paradoxical role for VEGF. Studies in animal models suggest that VEGF and its receptors play a protective role in the development of emphysema (Kanazawa et al. 2003; Kasahara et al. 2000). Increased VEGF concentrations in induced sputum in chronic bronchitis and enhanced bronchial expression of VEGF and its receptors on the other hand point on VEGF involvement in airway remodeling process in COPD (Kranenburg et al. 2005; Rovina et al. 2007).
Other growth factors involved in the angiogenesis process include angiogenin and IL-8. Angiogenin was first isolated from conditioned media from colonic carcinoma cell cultures (Fett et al. 1985; Polverini 1995). In vivo, angiogenin induces vascular endothelial cell proliferation, migration, and tubule formation (Kishimoto et al. 2005). IL-8, a chemokine that is produced by the bronchial epithelium and macrophages and is a potent neutrophil activator and chemoattractant, was found in elevated concentrations in the induced sputum of patients with COPD (Broide et al. 1992; Jatakanon et al. 1999; Mazzarella et al. 2000; Ordonez et al. 2000).
Angiogenesis also plays a role in asthma airway remodeling, where an imbalance in favor of angiogenic factors leads to the abnormal growth of new blood vessels (Puxeddu et al. 2005). In vivo results also suggest increased airway angiogenesis in patients with rhinitis without asthma as well as in corticosteroid-treated and well controlled asthma (Kristan et al. 2009).
In previous studies only the up-regulation of blood levels of VEGF has been reported during the acute exacerbation of COPD (Valipour et al. 2008). Our research group has confirmed elevated concentrations of VEGF in the airways of patients with COPD, but we have not found any additional elevation of angiogenic factors at the time of exacerbation of COPD (Kristan et al. 2012). Moreover, we also demonstrated a negative correlation between FEV1 and levels of VEGF, angiogenin, and IL-8. These results therefore showed the impact of angiogenic factors in the severity of airway obstruction and suggest the important role of angiogenic factors in the process of remodeling and angiogenesis in COPD. These results support continued investigation of the process of angiogenesis in COPD.
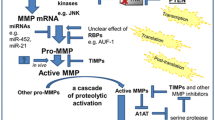
Biomarkers of Matrix Degradation
Desmosine
Elastic fibers are significant structural components of the skin, blood vessels, and lungs, where they provide physical recoil to distorting forces and contribute to normal physiological function (Mecham 1997). Elastin is the principle component of elastic fibers. In COPD, elastic fibers in emphysematous lungs appear distorted and fenestrated (Wright 1961). In the lungs and other structures, elastin is produced by smooth muscle cells, myofibroblasts, mesothelial cells, endothelial cells, and chondroblasts (Sandberg et al. 1981). Elastin synthesis involves secretion of tropoelastin into the extracellular space and then cross-linking of monomers by two amino acids, desmosine and isodesmosine (DI) (Foster and Curtiss 1990). The cross-linking transforms the tropoelastin precursor into the insoluble mature elastic fiber. DI occurs only in mature elastin and their presence in body fluids is a chemical indicator of the degradation of mature elastic fibers. Some turnover of mature body elastin occurs in normal adults, because low detectable levels of DI in plasma and urine (Ma et al. 2003) are usually present. DI has not been detected in induced sputum in healthy individuals, which suggests the stability of this bronchial and lower airway elastin (Ma et al. 2003).
The detection and measurement of DI were recognized early as a means of investigating the state of elastin degradation in disease (Darnule et al. 1982; Goldstein and Starcher 1978; Luisetti et al. 2008). The presence of these amino acids in sputum can be a consequence of elastin degradation in the lung. Measurements and detection of DI in sputum is possible with new analytical techniques (Ma et al. 2003). In previous studies the authors have demonstrated that smokers with normal lung function and patients with COPD excreted more desmosine in urine than healthy nonsmokers (Harel et al. 1980). Smokers who have stopped smoking had elevated excretion of desmosine but lower levels of excretion then those that continued to smoke (Stone et al. 1995). Gottlieb et al. (1996) showed that smokers with a more rapid decline of pulmonary function had higher levels of desmosine excretion than those with a slow decline of lung function.
A very recently published study by Huang et al. (2012) evaluated the validity of urinary and plasma total desmosine in patients with COPD and asthma and their relationship to exacerbation status and lung function. They showed that urinary desmosine levels are raised by exacerbation of COPD whereas blood desmosine levels are elevated in a subgroup of patients with stable COPD and reduced lung diffusing capacity. The authors speculate that a raised blood desmosine level may identify patients with increased elastin degradation suitable for targeted therapy. Despite being one of the most promising biomarkers linked to the pathophysiology of emphysema, the clinical validity and utility of urine and blood total desmosine as a biomarker for COPD remains unproven. The major reasons are factors related to the analytical validity of assays, small study groups, and the lack of large longitudinal studies to determine the predictive power of desmosine for patients’ clinical outcomes (Huang et al. 2012; Luisetti et al. 2008). As has already been stated in a paper by Turino et al. (2011) we therefore have the means of determining concentration and changes in concentration of DI determined by the overall state of multi-organ systemic inflammation as well as the level of degradation occurring in the elastin of the lung. Thus, this marker may be used as an indicator of changes in the state of inflammation as well as effects on lung elastin determined by the course of disease or by responses to therapy.
Conclusion
Research on biomarkers has been extremely active in recent years, because there is an urgent need to clarify the heterogeneity and natural history of COPD as a serious disease. This is an important approach and opportunity to develop new and useful therapeutic targets that might affect disease progression. With the exception of lung function tests, currently there are no well-validated biomarkers or surrogate endpoints that can be used to establish the efficacy of a drug for COPD.
In large part, biomarkers that can be measured from blood specimens reflect the state of systemic inflammation, which is influenced by comorbidities as well as the primary disease. Thus, how blood levels are related to the lung disease still requires clarification. Moreover, trials based on research on known clinical variables such as FEV1, BODE, and 6MWT in combination with biomarkers from lung and blood specimens will probably clarify a part of the prognosis and natural history of the disease. This will also represent an additional step in COPD phenotyping and new treatment possibilities.
References
(1980) Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive pulmonary disease: a clinical trial. Ann Intern Med 93:391–398
(1983) Intermittent positive pressure breathing therapy of chronic obstructive pulmonary disease: a clinical trial. Ann Intern Med 9:612–620
(1999) Pulmonary rehabilitation 1999. Statement of American Thoracic Society. Am J Respir Crit Care Med 159:1666–1682
Adnot S, Andrivet P, Chabrier PE (1989) Atrial natriuretic factor in chronic obstructive lung disease with pulmonary hypertension. Physiological correlates and response to peptide infusion. J Clin Invest 83:986–993
Agusti AG (2005) Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2:367–370
Agusti A (2007) Systemic effects of chronic obstructive pulmonary disease: what we know and what we don’t know (but should). Proc Am Thorac Soc 4:522–525
Agusti AG, Noguera A, Sauleda J et al (2003) Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 21:347–360
Agusti A, Edwards LD, Rennard SI et al (2012) Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS ONE 7:e37483
Alpert JS, Thygesen K, Antman E et al (2000) Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 36:959–969
Anthonisen NR, Connett JE, Kiley JP et al (1994) Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA 272:1497–1505
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166:111–117
Barnes PJ, Celli BR (2009) Systemic manifestations and comorbidities of COPD. Eur Respir J 33:1165–1185
Bernasconi M, Tamm M, Bingisser R et al (2011) Midregional proatrial natriuretic peptide predicts survival in exacerbations of COPD. Chest 140:91–99
Bhowmik A, Seemungal TA, Sapsford RJ et al (2000) Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 55:114–120
Broide DH, Lotz M, Cuomo AJ et al (1992) Cytokines in symptomatic asthma airways. J Allergy Clin Immunol 89:958–967
Bucher HC, Guyatt GH, Cook DJ et al (1999) Users’ guides to the medical literature: XIX. Applying clinical trial results. A. How to use an article measuring the effect of an intervention on surrogate end points: evidence-based medicine working group. JAMA 282:771–778
Calverley PM, Anderson JA, Celli B et al (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356:775–789
Castell JV, Gómez-Lechón MJ, David M et al (1989) Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett 242:237–239
Cazzola M, MacNee W, Martinez FJ et al (2008) Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 31:416–468
Celli BR (2010) Predictors of mortality in COPD. Respir Med 104:773–779
Celli BR, Cote CG, Jose M et al (2004) The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 350:1005–1012
Celli BR, Locantore N, Yates J et al (2012) Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 185:1065–1072
Chang MK, Binder CJ, Torzewski M et al (2002) C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci USA 99:13043–13048
Chang CL, Robinson SC, Mils GD et al (2011) Biochemical markers of cardiac dysfunction predict mortality in acute exacerbation of COPD. Thorax 66:764–768
Chaouat A, Naeije R, Weitzenblum E (2008) Pulmonary hypertension in COPD. Eur Respir J 32:1371–1385
Chatila WM, Thomashow BM, Minai OA et al (2008) Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc 5:549–555
Chomarat P, Banchereau J, Davoust J et al (2000) IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol 1:510–514
Dahl M, Nordestgaard BG (2009) Markers of early disease and prognosis in COPD. Int J Chron Obstruct Pulmon Dis 4:157–167
Dahl M, Tybjaerg-Hansen A, Vestbo J et al (2001) Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164:1008–1011
Dahl M, Vestbo J, Lange P et al (2007) C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175:250–255
Danesh J, Lewington S, Thomson SG et al (2005) Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 294:1799–1809
Darnule TV, McKee M, Darnule AT et al (1982) Solid-phase radioimmunoassay for estimation of elastin peptides in human sera. Anal Biochem 122:302–307
De Gruttola VG, Clax P et al (2001) Considerations in the evaluation of surrogate endpoints in clinical trials. Summary of a national institutes of health workshop. Control Clin Trials 22:485–502
de Lemos JA, Morrow DA, Bentley JH et al (2001) The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 345:1014–1021
de Torres JP, Cordoba-Lanus E, Lopez-Aguilar C et al (2006) C-relative protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J 27:902–907
Di Francia M, Barbier D, Mege JL et al (1994) Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 150:1453–1455
DiCosmo BF, Geba GP, Picarella D et al (1994) Airway epithelial cell expression of interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivity. J Clin Invest 94:2028–2035
Donaldson GC (2007) C-reactive protein. Does it predict mortality? Am J Respir Crit Care Med 175:209–210
Donaldson GC, Seemungal TA, Bhowmik A et al (2002) Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 57:847–852
Donaldson GC, Seemungal TA, Patel IS et al (2003) Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J 22:931–936
Donaldson GC, Seemungal TA, Patel IS et al (2005) Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 128:1995–2004
Eagan TML, Gabazza EC, Gabazza CA et al (2012) TNF-α is associated with loss of lean body mass only in already cachectic COPD patients. Respir Res 13:48
Espiner EA, Richards AM, Yandle TG et al (1995) Natriuretic hormones. Endocrinol Metab Clin North Am 24:481–509
Fabbri LM, Rabe KF (2007) From COPD to chronic systemic inflammatory syndrome? Lancet 370:797–799
Ferrara N (2000) VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol 11:617–624
Fett JW, Strydom DJ, Lobb RR et al (1985) Isolation and characterisation of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry 24:5480–5486
Foster JA, Curtiss SW (1990) The regulation of lung elastin synthesis. Am J Physiol 259(2 Pt 1):L13–L23
Fukuoka Y, Xia HZ, Sanchez-Muñoz LB et al (2008) Generation of anaphylatoxins by human beta-tryptase from C3, C4, and C5. J Immunol 180:6307–6316
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454
Gan WQ, Man SF, Senthilselvan A et al (2004) Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 59:574–580
Garcia-Aymerich J, Monso E, Marrades RM et al (2001) Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation: eFRAM study. Am J Respir Crit Care Med 164:1002–1007
Gerardi DA, Lovett L, Benoit-Connors ML et al (1996) Variables related to increased mortality following outpatient pulmonary rehabilitation. Eur Respir J 9:431–435
Gershov D, Kim S, Brot N et al (2000) C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an anti-inflammatory innate immune response: implications for systemic autoimmunity. J Exp Med 192:1353–1364
Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2011) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (revised)
Goldstein RA, Starcher BC (1978) Urinary excretion of elastin peptides containing desmosine after intratracheal injection of elastin in hamsters. J Clin Invest 61:1286–1290
Gottlieb DJ, Stone PJ, Sparrow D et al (1996) Urinary desmosine excretion in smokers with and without rapid decline of lung function: the Normative Aging Study. Am J Respir Crit Care Med 154:1290–1295
Gunther C, Bello-Fernandez C, Kopp T et al (2005) CCL18 is expressed in atopic dermatitis and mediates skin homing of human memory T cells. J Immunol 174:1723–1728
Hajiro T, Nishimura K, Tsukino M et al (1998) Comparison of discriminative properties among disease specific questionnaires for measuring health related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157:785–790
Harel S, Janoff A, Yu SY et al (1980) Desmosine radioimmunoassay for measuring elastin degradation in vivo. Am Rev Respir Dis 122:769–773
Harvey MG, Hancox RJ (2004) Elevation of cardiac troponins in exacerbation of chronic obstructive pulmonary disease. Emerg Med Australas 16:212–215
Hierholzer C, Kalff JC, Omert L et al (1998) Interleukin-6 production in hemorrhagic shock is accompanied by neutrophil recruitment and lung injury. Am J Physiol 275:L611–L621
Huang TJ, Chaudhuri R, Albarbarawi O et al (2012) Clinical validity of plasma and urinary desmosine as biomarkers for chronic obstructive pulmonary disease. Thorax 67:502–508
Hurst JR, Donaldson GC, Perera WR et al (2006) Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174:867–874
Jatakanon A, Uasuf C, Maziak W et al (1999) Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med 160:1532–1539
Johnston RA, Schwartzman IN, Flynt L et al (2005) Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol 288:L390–L397
Kanazawa H, Asai K, Hirata K et al (2003) Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med 114:354–358
Kaner RJ, Crystal RG (2001) Compartmentalization of vascular endothelial growth factor to the epithelial surface of the human lung. Mol Med 7:240–246
Kasahara Y, Tuder RM, Taraseviciene-Stewart L et al (2000) Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106:1311–1319
Kazi AS, Lotfi S, Goncharova EA et al (2004) Vascular endothelial growth factor-induced secretion of fibronectin is ERK dependent. Am J Physiol Lung Cell Mol Physiol 286:L539–L545
Kessler R, Faller M, Fourgaut G et al (1999) Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159:158–164
Kishimoto K, Liu S, Tsui T et al (2005) Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene 24:445–456
Kishore U, Greenhough TJ, Waters P et al (2006) Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol 43:1293–1315
Köhl J (2001) Anaphylatoxins and infectious and non-infectious inflammatory diseases. Mol Immunol 38:175–187
Kony S, Zureik M, Driss F et al (2004) Association of bronchial hyperresponsiveness and lung function with C-reactive protein (CRP): a population based study. Thorax 59:892–896
Kraaijeveld AO, de Jager SC, de Jager WJ et al (2007) CC chemokine ligand-5 (CCL5/RANTES) and CC chemokine ligand-18 (CCL18/PARC) are specific markers of refractory unstable angina pectoris and are transiently raised during severe ischemic symptoms. Circulation 116:1931–1941
Kragelund C, Gronning B, Kober L et al (2005) N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med 352:666–675
Kranenburg AR, de Boer WI, Alagappan VK et al (2005) Enhanced bronchial expression of vascular endothelial growth factor and receptors (Flk-1 and Flt-1) in patients with chronic obstructive pulmonary disease. Thorax 60:106–113
Kristan SS, Malovrh MM, Silar M et al (2009) Airway angiogenesis in patients with rhinitis and controlled asthma. Clin Exp Allergy 39:354–360
Kristan SS, Marc MM, Kern I et al (2012) Airway angiogenesis in stable and exacerbated chronic obstructive pulmonary disease. Scand J Immunol 75:109–114
Lainscak M, von Haehling S, Anker SD (2009) Natriuretic peptides and other biomarkers in chronic heart failure: from BNP, NT-proBNP, and MR-proANP to routine biochemical markers. Int J Cardiol 132:303–311
Lomas DA, Silverman EK, Edwards LD et al (2009) Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur Respir J 34:95–102
Loza MJ, Watt R, Baribaud F et al (2012) Systemic inflammatory profile and response to anti-tumor necrosis factor therapy in chronic obstructive pulmonary disease. Respir Res 13:12
Luisetti M, Ma S, Iadarola P et al (2008) Desmosine as a biomarker of elastin degradation in COPD: current status and future directions. Eur Respir J 32:1146–1157
Ma S, Lieberman S, Turino GM et al (2003) The detection and quantitation of free desmosine and isodesmosine in human urine and their peptide-bound forms in sputum. Proc Natl Acad Sci USA 100:12941–12943
Macie C, Wooldrage K, Manfreda J et al (2006) Inhaled corticosteroids and mortality in COPD. Chest 130:640–646
Mahajan N, Mehta Y, Rose M et al (2006) Elevated troponin level is not synonymous with myocardial infarction. Int J Cardiol 111:442–449
Mahler DA, Criner GJ (2007) Assessment tools for chronic obstructive pulmonary disease: do newer metrics allow for disease modification? Proc Am Thorac Soc 4:507–511
Mahler DA, Weinberg DH, Wells CK et al (1984) The measurement of dyspnea: contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 85:751–758
Malhotra S, Man SF, Sin DD (2006) Emerging drugs for the treatment of chronic obstructive pulmonary disease. Expert Opin Emerg Drugs 11:275–291
Man SF, Connett JE, Anthonisen NR et al (2006) C-reactive protein and mortality in mild to chronic obstructive pulmonary disease. Thorax 61:849–853
Marc MM, Korosec P, Kosnik M et al (2004) Complement factors c3a, c4a, and c5a in chronic obstructive pulmonary disease and asthma. Am J Respir Cell Mol Biol 31:216–219
Marc MM, Kristan SS, Rozman A et al (2010) Complement factor C5a in acute exacerbation of chronic obstructive pulmonary disease. Scand J Immunol 71:386–391
Marcun R, Sustic A, Brguljan PM et al (2012) Cardiac biomarkers predict outcome after hospitalisation for an acute exacerbation of chronic obstructive pulmonary disease. Int J Cardiol 161:156–159
Martin LD, Rochelle LG, Fischer BM et al (1997) Airway epithelium as an effector of inflammation: molecular regulation of secondary mediators. Eur Respir J 10:2139–2146
Mazzarella G, Grella E, D’Auria D et al (2000) Phenotypic features of alveolar monocytes/macrophages and IL-8 gene activation by IL-1 and TNF-a in asthmatic patients. Allergy 55(Suppl 61):36–41
Mecham RP (1997) Elastin fibers in the lung. In: Crystal RG, West JB, Weibel ER, Barnes PJ (eds) Scientific foundations. Lippincott-Raven, Philadelphia, pp 729–736
Mold C, Rodic-Polic B, Du Clos TW (2002) Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fc receptors. J Immunol 168:6375–6381
Mori K, Kurihara N, Hayashida S et al (2002) The intrauterine expression of surfactant protein D in the terminal airways of human fetuses compared with surfactant protein A. Eur J Pediatr 161:431–434
Mura M, dos Santos CC, Stewart D et al (2004) Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol 97:1605–1617
Nagaya N, Nishikimi T, Uematsu M et al (2000) Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation 102:865–870
Neas LM, Schwartz J (1998) Pulmonary function levels as predictors of mortality in a national sample of US adults. Am J Epidemiol 147:1011–1018
Nussbaumer-Ochsner Y, Rabe KF (2011) Systemic manifestations of COPD. Chest 139:165–173
Oostenbrink JB, Rutten-van Molken MP (2004) Resource use and risk factors in high-cost exacerbations of COPD. Respir Med 98:883–891
Ordonez CL, Shaughnessy TE, Matthay MA et al (2000) Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma. Am J Respir Crit Care Med 161:1185–1190
Papaioannou AI, Kostikas K, Kollia P et al (2006) Clinical implications for vascular endothelial growth factor in the lung: a friend or foe? Respir Res 7:128
Pauwels R, Buist A, Calverley P et al (2001) Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 163:1256–1276
Pinto-Plata VM, Mullerova H, Toso JF et al (2006) C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 61:23–28
Pinto-Plata V, Toso J, Lee K et al (2007) Profiling serum biomarkers in patients with COPD: associations with clinical parameters. Thorax 62:595–601
Polverini PJ (1995) The pathophysiology of angiogenesis. Crit Rev Oral Biol Med 6:230–247
Puxeddu I, Ribatti D, Crivellato E et al (2005) Mast cells and eosinophils: a novel link between inflammation and angiogenesis in allergic diseases. J Allergy Clin Immunol 116:531–536
Racki S, Zaputovic L, Mavric Z et al (2006) C-reactive protein is a strong predictor of mortality in hemodialysis patients. Ren Fail 28:427–433
Rennard SI, Fogarty C, Kelsen S et al (2007) The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175:926–934
Rovina N, Papapetropoulos A, Kollintza A et al (2007) Vascular endothelial growth factor reflecting airway inflammation in healthy smokers and in patients with bronchitis type of chronic obstructive pulmonary disease? Respir Res 8:53
Sandberg LB, Soskel NT, Leslie JG (1981) Elastin structure, biosynthesis, and relation to disease states. N Engl J Med 304:566–579
Sapey E, Bayley D, Ahmad A et al (2008) Interrelationships between inflammatory markers in patients with stable COPD with bronchitis: intra-patient and inter-patient variability. Thorax 63:493–499
Schols AM, Slangen J, Volovics L et al (1998) Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157:1791–1797
Seemungal TA, Donaldson GC, Bhowmik A et al (2000) Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Med 161:1608–1613
Sin DD, Vestbo J (2009) Biomarkers in obstructive pulmonary disease. Proc Am Thorac Soc 6:543–545
Sin DD, Lacy P, York E et al (2004) Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 170:760–765
Sin DD, Miller BE, Duvoix A (2011) Serum PARC/CCL-18 concentrations and health outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 183:1187–1192
Soler N, Torres A, Ewig S et al (1998) Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 157:1498–1505
Stone PJ, Gottlieb DJ, O’Connor GT et al (1995) Elastin and collagen degradation products in urine of smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 151:952–959
Sutherland ER, Cherniack RM (2004) Management of chronic obstructive pulmonary disease. N Engl J Med 350:2689–2697
Suwa T, Hogg JC, Klut ME et al (2001) Interleukin-6 changes deformability of neutrophils and induces their sequestration in the lung. Am J Respir Crit Care Med 163:970–976
Swedberg K, Cleland J, Dargie H et al (2005) Guidelines for the diagnosis and treatment of chronic heart failure: full text (update 2005). Eur Heart J 26:1115–1140
Thomason MJ, Strachan DP (2000) Which spirometric indices best predict subsequent death from chronic obstructive pulmonary disease? Thorax 55:785–788
Tulevski II, Hirsch A, Sanson BJ et al (2001) Increased brain natriuretic peptide as a marker for right ventricular dysfunction in acute pulmonary embolism. Thromb Haemost 86:1193–1196
Turino GM, Ma S, Lin YY et al (2011) Matrix elastin. A promising biomarker for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 184:637–641
US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2007) Guidance for industry chronic obstructive pulmonary disease: developing drugs for treatment. Available via http://www.fda.gov/CDER/GUIDANCE/7875dft.pdf. Accessed 14 April 2009
Valipour A, Schreder M, Wolzt M et al (2008) Circulating vascular endothelial growth factor and systemic inflammatory markers in patients with stable and exacerbated chronic obstructive pulmonary disease. Clin Sci 115:225–232
van Tits L, de Graaf J, Toenhake H et al (2005) C-reactive protein and annexin A5 bind to distinct sites of negatively charged phospholipids present in oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol 25:717–722
Vestbo J, Edvards LD, Scanlon PD et al (2011) Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 365:1184–1192
Vignola AM, Rennar SI, Hargreave FE et al (2002) Standardised methodology of sputum induction and processing: future directions. Eur Respir J Suppl 37:51s–55s
Walters EH, Reid D, Soltani A et al (2008) Angiogenesis: a potentially critical part of remodelling in chronic airway diseases? Pharmacol Ther 118:128–137
Weiser JN, Pan N, McGowan KL et al (1998) Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med 187:631–640
Wills-Karp M (2007) Complement activation pathways: a bridge between innate and adaptive immune responses in asthma. Proc Am Thorac Soc 4:247–251
Witte KK, Ford SJ, Preston T et al (2008) Fibrinogen synthesis is increased in cachectic patients with chronic heart failure. Int J Cardiol 129:363–367
World Health Organization (2008) Global burden of disease 2004: update part 2, causes of death. Available via http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_part2.pdf. Accessed 14 Feb 2012
Wouters EF (2006) The systemic face of airway diseases: the role of C-reactive protein. Eur Respir J 27:877–879
Wright RR (1961) Elastic tissue of normal and emphysematous lungs—a tridimensional histologic study. Am J Pathol 39:355–367
Acknowledgments
I would like to thank Mitja Košnik and Mitja Lainščak for their feedback on this paper.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kristan, S.S. Blood Specimen Biomarkers of Inflammation, Matrix Degradation, Angiogenesis, and Cardiac Involvement: a Future Useful Tool in Assessing Clinical Outcomes of COPD Patients in Clinical Practice?. Arch. Immunol. Ther. Exp. 61, 469–481 (2013). https://doi.org/10.1007/s00005-013-0237-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-013-0237-y