Abstract
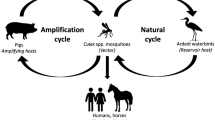
Japanese encephalitis is considered as a serious disease due to the complexity of the disease and lack of specific treatment. A secular trend towards declining of JE has been brought in China, Korea, and Japan with widespread use of JE vaccine. In India, the actual JE burden could be estimated only by strengthening diagnostic facilities for JE confirmation in hospitals. However, the available records at present indicate a rising trend in JE-occurrence and expansion of the disease into JE non-endemic areas, which cannot be ignored. JE control through vector control methods has limitations owing to sustainability and cost effectiveness of the programs. Under these circumstances, feasibility of JE vaccination in India has to be considered as a preventive measure, for which identification of risk areas, target populations to be immunized, cost-evaluation of immunization is emphasized. Since, JE vaccine is produced in India, extension of the availability of this vaccine into routine JE-immunization programs is not remote. China has proved that countries with limited sources can produce safe and effective JE vaccines.
Similar content being viewed by others
References
Japanese encephalitis:World Organization for Animal Health (OIE), 2003; 1–3.
Halstead SB. Arboviruses of the pacific and Southeast Asia. In Feigin RD, Cherry JD, eds.Textbook of Pediatric Infectious Diseases, 3rd edn. Philadelphia, PA: WB Saunders. 1992; 1468–1475.
Huy B, Tu H, Luan T, Lindqvist R. Early mental and neurological sequelae after Japanese B encephalitis.Am J Trop Med Hyg 1994; 25: 549–553.
Kabilan L, Edwin N, Balashankar S, Meikandan D. Japanese encephalitis among paediatric patients with acute encephalitis syndrome in Tamil Nadu, India.Trans Roy Soc Trop Med and Hyg 2000; 94: 157–158.
Tiroumourougane SV, Raghava P, Srinivasan S, Badrinath. Management parameters affecting the outcome of Japanese encephalitis.J Trop Pediatr 2003; 49(3): 153–156.
Raghava P, Badrinath S, Srinivasan S. Japanese encephalitis in and around Pondicherry, South India: A Clinical and prognostic indicators for the outcome.J Trop Pediatr 2003; 49(1): 48–53.
Mathur A, Tandon HO, Mathur KRet al. Japanese encephalitis infection during pregnancy.Indian J Med Res 1985; 81: 9–12.
Philip Samuel P. Japanese encephalitis in India with special reference to the surveillance tools and control measures.Recent Trends in Combating Mosquitoes 2000; 240–252. Proceeding of Loyola College, Chennai, October 3–4, 2000.
Lowry PW, Truong DH, Hinh LDet al. Japanese encephalitis among hospitalized pediatric and adult patients with acute encephalitis syndrome in Hanoi, Vietnam.Am J Trop Med and Hyg 1995; 58: 324–329.
Ayukawa R, Fujimoto H, Ayabe Met al. An unexpected outbreak of Japanese encephalitis in the Chugoku district of Japan.Jpn J Infect Dis 2002; 57: 63–66.
Gajanana A, Rajendran R, Philip Samuel Pet al. Japanese encephalitis in South Arcot district, Tamil Nadu: A three year longitudinal study of vector abundance and infection frequency.J Med Entomol 1996; 34(6): 651–659.
Rao DR, Reuben R, Nagasampagi BA. Development of combined use of neem (Azadirachta indica) and water management for the control of Culicine mosquitoes in rice fields.Med and Vet Entomol 1995; 9: 25–33.
John Victor T, Chandarasekaran R, Reuben R. Composite fish culture for mosquito control in rice fields in southern India. ’Southeast Asian J Trop Med and Pub Hlth 1994; 25: 522–527.
Rajendran R, Reuben R. Evaluation of the water fernAzolla microphylla for mosquito population management in the rice land agro ecosystem. /Med and Vet Entomol 1991; 5: 299–310.
Sundararaj R, Reuben R. Evaluation of a microgel droplet formulation ofBacillus sphaericus 1593M (Biocide-S) for control of mosquito larva in rice fields in Southern India.J Am Mos Con Assoc 1991; 7: 556–759.
Control of Japanese encephalitis using pyrethroid sprayed polypropylene curtain: entomological evaluation:Centre for Research in Medical Entomology, Annual Report 2001–2002,9–13.
Theodore F, Tsai, MJD, MPH. Japanese Encephalitis Vaccines. CDC, Fort Collins, USA, Pub: 1990.
Inactivated Japanese encephalitis virus vaccine. Recommendations of the Advisory committee on Immunization Practices (ACIP). CDC. MMWR 1993; 42: 1–22.
Oya, Akira. Japanese encephalitis vaccine. In H. Fukumi, ed.The Vaccination Theory and Practice, (International Medical Foundation of Japan, Japan) 1976; 69.
Rao Bhau LN, Singh, Gurpalet al. Safety and efficacy of Japanese encephalitis vaccine produced in India.IndianJ Med Res 1988; 88: 301.
Raengsakulrach B, Nisalak A, Gettayacamin Met al. Safety immunogenicity, and protective efficacy of NYVAC-JEV and ALVAC-JEV recombinant Japanese encephalitis vaccines in rhesus monkeys.Am J Trop Med Hyg 1999; 60(3): 343–349.
Lai CJ, Monath TP. Chimeric flaviviruses: novel vaccines against dengue fever, tick-borne encephalitis, and Japanese encephalitis.Adv Virus Res 2003; 61: 469–509.
Monath TP, Soike K, Levenbook Iet al. Recombinant, chimaeric live, attenuated vaccine (ChimeriVax) incorporating the envelope genes of Japanese encephalitis (SA14-14-2) virus and the capsid and nonstructural genes of yellow fever (17D) virus is safe, immunogenic and protective in non-human primates.Vaccine 1999; 17(15–16): 1869–1882.
Tanabayashi K, Mukai R, YamActa Aet al. Immunogenicity of a Japanese encephalitis DNA vaccine candidate in cynomolgus monkeys.Vaccine 2003; 21(19–20): 2338–2345.
Konishi E, Yamaoka M, Win Ket al. Induction of protective immunity against Japanese encephalitis in mice by immunization with a plasmid encoding Japanese encephalitis virus premembrane and envelope genes.J Virol 1998, 72(6): 4925–4930.
Kaur R, Vrati S. Development of a recombinant vaccine against Japanese encephalitis.J Neurovirol 2003; 9: 421–431.
Regional Antiepidemic Station, Hueyang, Guangtong: Preliminary observation on epidemiological effectiveness of JE live vaccine.Bull Biological Products 1978; 7: 111–114.
Saxena SN, Rao Bhau LN, Gurupal Singhet al. Immune status of volunteers one year after administration of Japanese encephalitis vaccine produced in India.Indian J Med Res 1989; 89: 362–367.
Mohan Rao CVR, Risbud AR, Dandawate CNet al. Serological response to Japanese encephalitis vaccine in a group of school children in South Arcot district of Tamil Nadu.Indian J Med Res 1993; 97: 53–59.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kabilan, L. Control of Japanese encephalitis in India: A reality. Indian J Pediatr 71, 707–712 (2004). https://doi.org/10.1007/BF02730659
Issue Date:
DOI: https://doi.org/10.1007/BF02730659