Abstract
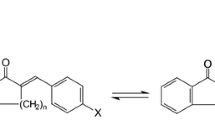
Gas chromatography (GC)-electron ionization mass spectrometry of 2-alkenyl-4,4-dimethyl-oxazoline derivatives was used to confirm the identities of a complex mixture of C18 diunsaturated cyclic fatty acid monomers (CFAMs) that were isolated from heated flaxseed (linseed) oil. The positions of double bonds and 1,2-disubstituted unsaturated 5- and 6-membered rings along the fatty acid hydrocarbon chains were established by this method. The oxazoline spectra exhibited a homologous ion series with a pattern of peaks that were 14 u (u=atomic mass unit) apart but interrupted when a double bond (12-u mass interval) or a ring was present along the fatty acid chain. The identity and location of a ring were indicated by a large interval of 68, 82, 66, 80, 78, or 120 u for a saturated 5- or 6-membered ring, monounsaturated 5- or 6-membered ring, diunsaturated 6-membered ring, or monounsaturated bicyclic ring system (fused 5- and 6-membered rings), respectively. The double bond configuration for the methyl ester derivatives of these CFAMs was established by GC-matrix isolation-Fourier transform infrared spectroscopy. The elucidated alkenyl structures at C2 in diunsaturated 2-[alkenyl]-4,4-dimethyloxazolines were 8-(2-but-trans-1-enyl-cyclopentenyl)octyl, 9-(2-propyl-cyclopentenyl)non-rans-8-enyl, 9-(2-propyl-cyclopentenyl)non-cis-7-enyl, 8-(2-but-cis-1-enyl-cyclopentenyl)ocytl, 9-(2-propylcyclopentenyl)non-cis-8-enyl, 8-(2-propyl-cyclohex-cis-4-enyl)oct-trans-7-enyl, 8-(prop-trans-1-enyl-cyclohex-cis-4-enyl)ocytl, and 8-(2-propylcyclohexa-cis,cis-3,5-dienyl)octyl.
Similar content being viewed by others
References
Sebedio, J.L., and A. Grandgirard,Prog. Lipid Res. 28:303 (1989).
Hutchison, R.B., and J.C. Alexander,J. Org. Chem. 28:2522 (1963).
Friedrich, J.P.,J. Am. Oil Chem. Soc. 44:244 (1967).
Graille, J., A. Bonfand, P. Perfetti and M. Naudet,Chem. Phys. Lipids 27:23 (1980).
Awl, R.A., and E.N. Frankel,Lipids 17:414 (1982).
Vatele, J.M., J.-L. Sebedio and J.L. Le Quere,Chem. Phys. Lipids 48:119 (1988).
Rojo, J.A., and E.G. Perkins,J. Am. Oil Chem. Soc. 66:1593 (1989).
Le Quere, J.L., J.-L. Sebedio, R. Henry, F. Couderc, N. Demont and J.C. Prome,J. Chromatogr. 562:659 (1991).
MacDonald, J.A.,J. Am. Oil Chem. Soc. 33:394 (1956).
McInnes, A.G., F.P. Cooper and J.A. MacDonald,Can. J. Chem. 39:1906 (1961).
Gast, L.E., W.J. Schneider, C.A. Forest and J.C. Cowan,J. Am. Oil Chem. Soc. 40:287 (1963).
Saito, M., and T. Kaneda,Yukagaku 25:79 (1976).
Potteau, B., P. Dubois and J. Rigaud,Ann. Technol. Agric. 27:655 (1978).
Sebedio, J.L., J. Prevost and A. Grandgirard,J. Am. Oil Chem. Soc. 64:1026 (1987).
Rojo, J.A., and E.G. Perkins, Ibid.:414 (1987).
Christie, W.W., E.Y. Brechany, J.-L. Sebedio and J.L. Le Quere,Chem. Phys. Lipids 66:143 (1993).
Christie, W.W., E.Y. Brechany and V.K.S. Shukla,Lipids 24:116 (1989).
Sebedio, J.-L., J. Prevost, E. Ribot and A. Grandgirard,J. Chromatogr. A659:101 (1994).
Sebedio, J.-L., J.L. Le Quere, O. Morin, J.M. Vatele and A. Grandgirard,J. Am. Oil Chem. Soc. 66:704 (1989).
Zhang, J.Y., Q.T. Yu, B.N. Liu and Z.H. Huang,Biomed. Environ. Mass Spectrom. 15:33 (1988).
Yu, Q.T., B.N. Liu, J.Y. Zhang and Z.H. Huang,Lipids 24:804 (1989).
Zhang, J.Y., N.Y. Wang, Q.T. Yu, X.J. Yu, B.N. Liu and Z.H. Huang,J. Am. Oil Chem. Soc. 66:242 (1989).
Mossoba, M.M., M.P. Yurawecz, H.S. Lin, J.A.G. Roach, R.E. McDonald, B.D. Flickinger and E.G. Perkins,INFORM 4:514 (1993).
Mossoba, M.M., M.P. Yurawecz, J.A.G. Roach, H.S. Lin, R.E. McDonald, B.D. Flickinger and E.G. Perkins,Lipids 29:893 (1994).
Bourne, S., G. Reedy, P. Coffey and D. Mattson,Am. Lab. (Fairfield, Conn.) 160:90 (1984).
Reedy, G.T., D.C. Ettinger, J.F. Schneider and S. Bourne,Anal. Chem. 57:1602 (1985).
Mossoba, M.M., R.A. Niemann and J.-Y.T. Chen, Ibid.:1678 (1989).
Fay, L., and U. Richly,J. Chromatogr. 541:89 (1991).
Sebedio, J.-L., J.L. Le Quere, E. Semon, O. Morin, J. Prevost and A. Grandgirard,J. Am. Oil Chem. Soc. 64:1324 (1987).
Rojo, J.A., and E.G. Perkins,Lipids 24:467 (1989).
Mossoba, M.M., R.E. McDonald, J.-Y.T. Chen, D.J. Armstrong and S.W. Page,J. Agric. Food Chem. 38:86 (1990).
Pouchert, C.J. (ed.),Aldrich Library of Infrared Spectra, 3rd edn., Aldrich Chemical Company, Milwaukee, 1981.
Andersson, B.A., and R.T. Holman,Lipids 9:185 (1974).
Mikusch, J.D., and A.N. Sagredos,Fette Seifen Anstrichm. 73:384 (1971).
Hase, A., S. Kaltia, J. Matikainen, M. Ala-Peijari and T. Hase,J. Am. Oil Chem. Soc. 69:1027 (1992).
Author information
Authors and Affiliations
Additional information
Physical science aide, 1991–1992; currently a medical student at the University of Maryland, Baltimore, MD.
About this article
Cite this article
Mossoba, M.M., Yurawecz, M.P., Roach, J.A.G. et al. Elucidation of cyclic fatty acid monomer structures. Cyclic and bicyclic ring sizes and double bond position and configuration. J Am Oil Chem Soc 72, 721–727 (1995). https://doi.org/10.1007/BF02635662
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02635662