Summary
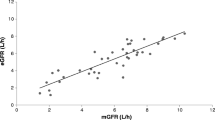
Plasma and urine levels of 12 healthy subjects and 30 patients with renal insufficiency of different degrees were examined after oral administration of four 250 mg capsules azithromycin (total daily dose 1,000 mg). The concentrations were determined by cup plate method. The pharmacokinetic parameters were determined model-dependent and noncompartmentally. Neither the area under the plasma concentration curve nor the distribution volume in steady state (16 l/kg body weight) nor the maximal plasma concentration were significantly affected by renal insufficiency. Thus the dosage regimen of azithromycin in renal impairment may (and should) be the same as in patients with normal renal function. The nonrenal clearance is not affected by renal insufficiency, but the concentration of the substance in the tubular lumen (the “tubular load”) may be increased.
Zusammenfassung
Plasma- und Urinspiegel von Azithromycin wurden bei 12 Normalpersonen und bei 30 Patienten mit Niereninsuffizienz verschiedenen Grades untersucht. Die Probanden hatten zuvor vier Kapseln zu 250 mg Azithromycin erhalten. Die Bestimmung erfolgte mittels des Platten-Loch-Testes. Die pharmakokinetischen Parameter wurden Modell-abhängig und Modell-unabhängig ermittelt. Weder die Fläche unter der Kurve (AUC) noch das Verteilungsvolumen im Steady State (16 l/kg Körpergewicht) noch die maximale Plasmakonzentration war bei Niereninsuffizienz signifikant verändert. Folglich kann und muß die Dosierung von Azithromycin bei Niereninsuffizienz genauso erfolgen wie bei Patienten mit normaler Nierenfunktion. Die nichtrenale Clearance ist bei Niereninsuffizienz nicht verändert, jedoch ist die Konzentration der Substanz im Tubuluslumen (das tubuläre load) erhöht.
Similar content being viewed by others
References
Peters, D. H., Friedel, H. A., McTavish, D Focus on azithromycin, D. Drug 44 no. 5 (1992) 750–799.
Foulds, G., Chan, K. H., Johnson, J. T., Shepard, R. M., Johnson, R. B. Concentrations of azithromycin in human tonsillar tissue. Eur. J. Clin. Microbiol. Infect. Dis. 10 (1991) 853–856.
Karma, P., Pukander, J., Penttilä, M. Azithromycin concentration in sinus fluid and mucosa after oral administration. Eur. J. Clin. Microbiol. Infect. Dis. 10 (1991) 856–859.
Foulds, G., Madsen, P., Cox, C., Shepard, R., Johnson, R. Concentrations of azithromycin in human prostatic tissue. Eur. J. Clin. Microbiol. Infect. Dis. 10 (1991) 868–871.
Morris, D. L., Souza, A. de, Jones, J. A. Morgan, W. E. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur. J. Clin. Microbiol. Infect. Dis. 10 (1991) 859–861.
Foulds, G.: Pharmacokinetics of azithromycin. Poster presented at the 6th International Congress of Infectious Diseases, Montreal 1990 a.
Riedel, K.-D., Wildfeuer, A., Laufen, H., Zimmermann, T. Equivalence of a high-performance liquid chromatographic assay and a bioassay of azithromycin in human serum samples. J. Chromatogr. 576 (1992) 358–362.
Fiegel, P., Höffler, D. The measurement of reduced glomerular filtration rate with the51Cr-EDTA single shot clearance. In:Kluthe, R., Berlyne, G., Burton, B. (eds.): Uremia, Annual International Conference on Pathogenesis, Diagnosis and Therapy. Georg Thieme, Stuttgart 1972.
Koeppe, P., Hamann, C. A program for non-linear regression analysis to be used on desk-top computers. Comput. Programs Biomed. 12 (1980) 121–125.
Benet, L. Z., Massoud, N., Gambertoglo, J. G. Pharmacokinetic basis for drug treatment. Raven, New York 1984.
Wildfeuer, A., Laufen, H., Leitold, M., Zimmermann, T. Comparison of the pharmacokinetics of three-day and five-day regimens of azithromycin in plasma and urine. J. Antimicrob. Chemother. 31 (Suppl. E) (1993) 51–56.
Bergan, T., Jorgensen, N. P., Olszewski, W., Zhang, Y. Azi-azithromycin pharmacokinetics and penetration to lymph. Scand. J. Infect. Dis. (Suppl.) 83 (1992) 15–21.
Foulds, G., Separd, R. M., Johnson, R. B. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25 (Suppl. A) (1990 b) 73–82.
Höffler, D., Koeppe, P., Williams, K. J. The pharmacokinetics of ceftazidime in normal and impaired renal function. J. Antimicrob. Chemother. 12 (Suppl. A) (1983) 241–245.
Höffler, D., Koeppe, P. Pharmacokinetics of cefotiam i.v in subjects with normal and impaired renal function. Acta Therapeutica 15 (1989) 3–16.
Höffler, D., Rotter-Tumpach, W., Koeppe, P., Wiese, K. Pharmacokinetics of SCE-2787, a new cephalosporin for intravenous administration in healthy subjects and patients with renal failure. Acta Therapeutica 18 (1992) 117–132.
Höffler, D., Koeppe, P., Häfner, F. Zur Pharmakokinetik von Piperacillin und Tazobactam bei normaler und eingeschränkter Nierenfunktion. Fortschr. Antimikr. Antineopl. Th. (FAC) 11 (4) (1992) 437–444.
Schentag, J., Ballow, C. H. Tissue-directed pharmacokinetics. Am. J. Med. 91 (Suppl. 3A) (1991) 3A-5S–3A-11S.
Harrison, J. D., Jones, J. A., Morris, D. L. Azithromycin levels in plasma and gastric tissue, juice and mucus. Eur. J. Clin. Microbiol. Infect. Dis. 10 (1991) 862–864.
Krohn, K. Gynaecological tissue levels of azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 10 (1991) 864–868.
Lode, H. The pharmacokinetics of azithromycin and their clinical significance. Eur. J. Clin. Microbiol. Infect. Dis. 10 (1991) 807–812.
Höffler, D., Koeppe, P. Nonrenal clearance and tubular load in renal failure. Arzneimittelforschung/Drug Res. 43 (III) (1993) 1233–1238.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Höffler, D., Paeske, B. & Koeppe, P. Pharmacokinetics of azithromycin in normal and impaired renal function. Infection 23, 356–361 (1995). https://doi.org/10.1007/BF01713565
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01713565