Abstract
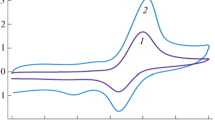
Dimenhydrinate exhibits a single adsorptive stripping peak at a hanging mercury drop electrode after accumulation at 0.0V vs Ag/AgCl electrode at pH 3.8 (acetate buffer). The addition of trace amounts of copper ions enhanced the dimenhydrinate peak and its height depends on the concentration of each dimenhydrinate and Cu2+. The adsorptive stripping response was evaluated with respect to accumulation time and potential, concentration dependence, electrolyte, the presence of other purines, surfactants and other metal ions, and some variables. The calibration graph for dimenhydrinate determination is linear over the range 2.0×10−8−2.0×10−7 M (pre-concentration for 60s). The correlation factor is found to be 0.985 and RSD is 3.2% at 1.0×10−7 M. Detection limit is 1.0×10−8 M after 5 min accumulation. The determination of dimenhydrinate in pharmaceutical formulations by the proposed method is also reported.
Similar content being viewed by others
References
“BritishPharmacopoeia” Office of the British Pharmacopoeia Commission, London, 1993.
F. Delaye, M. D. Gaye, J. J. Aaron,Anal. Chim. Acta 1989,223, 395.
V. Sustacek, F. Foret, P. Bocek,J. Chromatogr. 1989,480, 271.
J. Bohacek, B. Hosek, O. Babusikova, and A. Hrivankova,J. Chromatogr. B. Biomed. Appl. 1988,78, 439.
A. M. Tacchi-Bedford,J. Chromatogr. 1988,455, 364.
W. G. Stillwell, H. X. Xu, J. A. Adkins, J. S. Wishnok, S. R. Tannenbaum,Chem. Res. Toxicol. 1989,2, 94.
M. Katayama, H. Taniguchi,Talanta 1989,36, 117.
M. A. T. Gilmartin, J. P. Hart,Analyst 1992,117, 1613.
S. Xiang, C. Yumei, C. Hongyuan,Microchem. J. 1993,47, 287.
E. Palecek,Anal. Biochem. 1980,108, 129.
E. Palecek, F. Jelen, J. Lasovsky,Bioelectrochem. Bioenerg. 1981,8, 621.
J. C. Moreira, A. G. Fogg,Analyst 1990,115, 41.
J. C. Moreira, R. Zhao, A. G. Fogg,Analyst 1990,115, 1561.
U. Forsman,Anal. Chim. Acta 1983,146, 71.
S. Glodowski, R. Bilewicz, Z. Kublik,Anal. Chim. Acta 1986,186, 39.
E. Laviron,J. Electroanal. Chem. 1974,52, 35.
B. C. Househam, C. M. G. van den Berg, J. P. Riley,Anal. Chim. Acta 1987,200, 291.
A. G. Fogg, F. N. Ertas, J. C. Moreira, J. Bocek,Anal. Chim. Acta 1993,278, 41.
H. Shiraishi, R. Takahashi,Bioelectrochem. Bioenerg. 1993,31, 203.
R. M. Shubietah, A. Z. Abu Zuhri, A. G. Fogg,Anal. Lett. 1994,29(6), 1123.
R. M. Shubietah, PhD thesis,Loughborough University of Technology, LE11 3TU, UK, 1995.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shubietah, R.M., Abu Zuhri, A.Z. & Fogg, A.G. Adsorptive stripping voltammetric determination of dimenhydrinate at a hanging mercury drop electrode. Mikrochim Acta 130, 165–171 (1999). https://doi.org/10.1007/BF01244923
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01244923