Summary
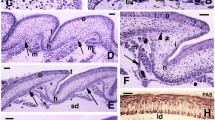
Collagen types I and III were purified from the skin of 3-or 7-week-old chickens, collagen type IV from bovine skin or EHS mouse tumour, fibronectin from human serum, and laminin from EHS mouse tumour. Antibodies were produced in rabbits or sheep, and used in indirect immunofluorescence on frozen sections of 9-to 16-day-old normal or mutant (scaleless) chick-embryo foot skin. In normal scale-forming skin and inscaleless skin, the distribution of anti-laminin and anti-type IV collagen label was uniform along the dermal-epidermal junction and showed no stage-related variations, except for fluorescent granules located in the dermis of early scale rudiments. By contrast, in normal scale-forming skin, the density of anti-types I and III label decreased in the dermis within scale rudiments, whereas it gradually increased in interscale skin. Conversely, anti-fibronectin label accumulated at a higher density within scale rudiments than in interscale skin. In the dermis of thescaleless mutant, anti-types I and III label and antifibronectin label were distributed evenly: the density of anti-collagen label increased with age, while that of antifibronectin decreased and almost completely vanished in 16-day-old skin, except around blood vessels. The microheterogeneous distribution of some extracellular matrix components, namely interstitial collagen types I and III and fibronectin, is interpreted as part of the morphogenetic message that the dermis is known to transmit to the epidermis during the formation of scales. The even distribution of these components in mutantscaleless skin is in agreement with this view. Basement membrane constituents laminin and type-IV collagen do not appear to be part of the dermal morphogenetic message.
Similar content being viewed by others
References
Abbott UK, Asmundson VS (1957) Scaleless, an inherited ectodermal defect in the domestic fowl. J Hered 48:63–70
Bernfield MR (1981) Organization and remodeling of the extracellular matrix in morphogenesis. In: Connely TG, Brinkley LL, Carlson BM (eds) Morphogenesis and pattern formation. Raven Press, New York, pp 139–162
Bernfield MR, Banerjee SD (1978) The basal lamina in epithelialmesenchymal morphogenesis interactions. In: Kefalides NA (ed) Proceeding, First International Symposium on the Biology and Chemistry of Basement Membranes. Academic Press, New York, pp 137–148
Bernfield MR, Wessels NK (1970) Intra-and extracellular control of epithelial morphogenesis. Dev Biol (suppl) 4:195–249
Bride M, Benslimane S, Stocker S, Grimaud JA (1982) Mise en évidence du collagène par immunofluorescence au cours du développement du Xénope, Xenopus laevis Daud. CR Soc Biol 176:494–502
Briggaman RA (1982) Biochemical composition of the epidermaldermal junction and other basement membrane. J Invest Dermatol 78: 1–6
Critchley DR, England MA, Wakely J, Hynes RO (1979) Distribution of fibronectin in the ectoderm of gastrulating chick embryos. Nature 280:498–500
David G, Bernfield MR (1979) Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: a possible mechanism for basal lamina formation. Proc Natl Acad Sci US Biol Sci 76:786–790
David G, Bernfield MR (1981) Type I collagen reduces the degradation of lamina proteoglycan by mammary epithelial cells. J Cell Biol 91:281–286
Demarchez M, Mauger A, Sengel P (1981) The dermal-epidermal junction during the development of skin and cutaneous appendages in the chick embryo. Arch Anat Microsc Morphol Exp 70:206–218
Ekblom P, Alitalo K, Vaheri A, Timpl R, Saxen L (1980) Induction of a basement membrane glycoprotein in embryonic kidney: possible role of laminin in morphogenesis. Proc Natl Acad Sci 77:485–489
Foidart JM, Reddi AH (1980) Immunofluorescent localization of type IV collagen and laminin during endochondral bone differentiation and regulation by pituary growth hormone. Dev Biol 75:130–136
Goetinck PF, Abbott UK (1963) Tissue interaction in scaleless mutant and the use of scaleless as an ectodermal marker in studies of normal limb differentiation. J Exp Zool 154:7–19
Greenberg JH, Seppä S, Seppä H, Hewitt AT (1981) Role of collagen and fibronectin in neural crest cell adhesion and migration. Dev Biol 87:259–266
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Hynes RO (1981) Fibronectin and its relation to cellular structure and behavior. In: Hay ED (ed) Cell biology of extracellular matrix. Academic Press, New York, pp 295–334
Kitamura K (1981) Distribution of endogenous β-galactoside-specific lectin, fibronectin and type I and III collagens during dermal condensation in chick embryos. J Embryol Exp Morphol 65:41–56
Kollar EJ, Baird GR (1970) Tissue interactions in embryonic mouse tooth germs. II. The inductive role of the dental papilla. J Embryol Exp Morphol 24:173–186
Lesot H, Osman M, Ruch JV (1981) Immunofluorescent localization of collagens, fibronectin and laminin during terminal differentiation of odontoblasts. Dev Biol 82:371–381
Lucas AM, Stettenheim PR (1972) Avian anatomy integument, Part II. In: Agriculture Handbook 362. US Government Printing Office. Washington DC, pp 341–721
Mauger A, Demarchez M, Georges D, Herbage D, Grimaud JA, Druget M, Hartmann DJ, Sengel P (1982a) Répartition du collagène, de la fibronectine et de la laminine au cours de la morphogenèse de la peau et des phanères chez l'embryon de Poulet. CR Acad Sci Paris 294 (Sér III) 475–480
Mauger A, Demarchez M, Herbage D, Grimaud JA, Druguet M, Hartmann D, Sengel P (1982b) Immunofluorescent localization of collagen types I and III, and of fibronectin during feather morphogenesis in the chick embryo. Dev Biol 94:93–105
Mayer BW, Hay ED, Hynes RO (1981) Immunocytochemical localization of fibronectin in embryonic chick trunk and area vasculosa. Dev Biol 82:267–286
Meier S, Hay ED (1974) Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Dev Biol 38:249–270
Melnick M, Jaskoll T, Brownell AG, Mac Dougall M, Bessem C, Slavkin HC (1981) Spatiotemporal patterns of fibronectin distribution during embryonic development. I. Chick limbs. J Embryol Exp Morphol 63:193–206
Newgreen D, Thiéry JP (1980) Fibronectin in early avian embryos: synthesis and distribution along the migration pathways of neural crest cells. Cell Tissue Res 211:269–291
Newman SA, Frisch HL (1979) Dynamics of skeletal pattern formation in developing chick limb. Science 205:662–668
Newman SA, Frisch HL, Perle MA, Tomasek JJ (1981) Limb development: Aspects of differentiation, pattern formation and morphogenesis. In: Morphogenesis and pattern formation (Connelly TG, Brinkley LL, Carlson BM (eds) Raven Press, New York, pp 163–178
Sawyer RH (1972) Avian scale development. I. Histogenesis and morphogenesis of the epidermis and dermis during formation of the scale ridge. J Exp Zool 181:365–384
Sawyer RH, Abbott UK (1972) Defective histogenesis and morphogenesis in the anterior shank skin of the scaleless mutant. J Exp Zool 181:99–110
Sawyer RH, Craig KF (1977) Avian scale development. Absence of an “epidermal placode” in reticulate scale morphogenesis. J Morphol 154:83–94
Sengel P (1976) Morphogenesis of skin. In: Abercrombie M, Newth DR, Toorey JG (eds) Developmental and cell biology series. Cambridge University Press, Cambridge, London, New York, Melbourne, pp 1–277
Sengel P, Abbott UK (1963) In vitro studies with the scaleless mutant: Interactions during feather and scale differentiation. J Hered 54:254–262
Sengel P, Dhouailly D, Mauger A (1980) Region-specific determination of epidermal differentiation in amniotes. In: Spearman RIC, Riley PA (eds) The skin of vertebrates. Academic Press, London, pp 185–200
Schor SL, Court J (1979) Different mechanisms in the attachment of cells to native and denatured collagen. J Cell Sci 38:267–281
Slavkin HC, Bavetta LA (1968) Odontogenic epithelio-mesenchymal interactions in vitro. J Dent Res 47:779–785
Spooner BR, Faubion JM (1980) Collagen involvement in branching morphogenesis of embryonic lung and salivary gland. Dev Biol 77:84–102
Stuart ES, Moscona AA (1967) Embryonic morphogenesis: role of fibrous lattice in the development of feathers and feather patterns. Science 157:947–848
Sturat ES, Garber B, Moscona AA (1972) An analysis of feather germ formation in the embryo and in vitro, in normal development and in skin treated with hydrocortisone. J Exp Zool 179:87–118
Terranova VP, Rohrbach DH, Martin GR (1980) Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell 22:719–726
Thesleff I (1978) Role of the basement membrane in odontoblast differentiation. J Biol buccale 6:241–249
Thesleff I, Barrach HJ, Foidart JM, Vaheri A, Pratt RM, Martin GR (1981) Changes in the distribution of type IV collagen, laminin, proteoglycan, and fibronectin during mouse tooth development. Dev Biol 81:182–192
Timpl R, Glanville RW, Wick G, Martin GR (1979a) Immunochemical study on basement membrane (type IV) collagens. Immunology 38:109–116
Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR (1979b) Laminin. A glycoprotein from basement membranes. J Biol Chem 254:9933–9937
Tomasek JJ, Mazurkiewicz JE, Newman SA (1982) Non uniform distribution of fibronectin during avian limb development. Dev Biol 90:118–126
Yamada KM (1981) Fibronectin and other structural proteins: In: Hay ED (ed) Cell biology of extracellular matrix. Academic Press, New York, pp 95–114
Yaoita H, Foidart JM, Katz SI (1978) Localization of the collagenous component in skin basement membrane. J Invest Deamatol 70:191–193
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mauger, A., Demarchez, M., Herbage, D. et al. Immunofluorescent localization of collagen types I, III, IV, fibronectin and laminin during morphogenesis of scales and scaleless skin in the chick embryo. Wilhelm Roux' Archiv 192, 205–215 (1983). https://doi.org/10.1007/BF00848651
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00848651