Abstract
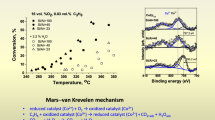
The decomposition of nitrous oxide on several Co- and Cu-ZSM-5 zeolite catalysts was studied in the absence and presence of excess oxygen. Also, the effect of methane addition, as well as catalyst steaming in dry and wet feeds is reported. N2O decomposition with no oxygen in the feed was proportional to metal loading on both catalysts. Co-ZSM-5 was much more resistant than Cu-ZSM-5 in excess oxygen. The tolerance of Co-ZSM-5 catalysts to excessive amounts of oxygen is high when Co2+ is stabilized in the zeolite framework and depends on the catalyst method of preparation. The presence of methane with no oxygen in the feed enhanced N2O decomposition while the addition of both methane and oxygen to the feed decreased N2O conversion on all catalysts tested. Co2+ ions stabilized by ZSM-5 framework have high hydrothermal stability in comparison to Cu2+ -exchanged ZSM-5.
Similar content being viewed by others
References
M.A. Wójtowicz, J.R. Pels and J.A. Moulijn, Fuel Process. Technol. 34 (1993) 1.
P.J. Crutzen, in: B. Bolin and R.B. Cook, eds. (Wiley, New York, 1983) pp. 67–112.
J.W. Elkins and R. Rossen, Summary Report 1988: Geophysical monitoring for climatic change, NOAAERL, Boulder (1989).
J.T. Houghton, G.J. Jenkins and J.J. Ephraums, eds.,Climate Change, The IPCC Scientific Assessment (Press Syndicate of the University of Cambridge, London, 1990).
Y. Li and J.N. Armor, Appl. Catal. B 1 (1992) L21.
Y. Li and J.N. Armor, Appl. Catal. B 3 (1993) 55.
V.P. Shiralkar and A. Clearfield, Zeolites 9 (1983) 363.
Y. Li and J.N. Armor, Appl. Catal. B 2 (1993) 239.
G.I. Panov, V.Y. Sovolev and A.S. Kharitonov, J. Mol. Catal. 61 (1990) 85.
T. Tabata, M. Kokitsu, O. Okada, T. Nakayama, Y. Yasumatsu and H. Sakane, in:Catalyst Deactivation, Stud. Surf. Sci. Catal., Vol. 84, eds. B. Delmon and G.F. Froment (Elsevier, Amsterdam, 1994) p. 410.
J. Valyon and K. Hall, J. Catal. 143 (1993) 520.
C.M. Fu, V.N. Korchak and W.K. Hall, J. Catal. 68 (1981) 166.
J. Leglise, J.O. Petunchi and W.K. Hall, J. Catal. 86 (1984) 392.
E.R.S. Winter, J. Catal. 15 (1969) 144.
E.R.S. Winter, J. Catal. 19 (1970) 32.
G.M. Dhar and V. Srinivasan, Int. J. Chem. Kinet. 14 (1982) 415.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Correa, C.M., Villa, A.L. & Zapata, M. Decomposition of nitrous oxide in excess oxygen over Co- and Cu-exchanged MFI zeolites. Catal Lett 38, 27–32 (1996). https://doi.org/10.1007/BF00806895
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00806895