Abstract
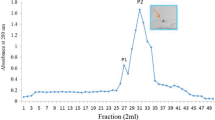
A lectin specific for glucosamine oligomers has been purified by chitosan affinity chromatography from cultured cells of Rubus. The lectin, eluted by a glucosamine oligomer of degree of polymerization 4 in the presence of l-α-phosphatidylserine dipalmitoyl, was found by sodium dodecyl sulfate-polyacrylamide gel electroploresis to be homogeneous and to have a molecular weight of 67 kilodaltons; it could best bind the tetrasaccharide, as shown by ligand-blot processing. Data from kinetic-dependent enzyme-linked immunosorbent assays showed that the lectin has two apparent binding sites which better accommodate the tetrasaccharide and the hexasaccharide, respectively, of the glucosamineoligomer series. The affinity of the lectin for glucosamine oligomers was shown to decrease for chain lengths greater than six glucosaminyl residues.
Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- DP:
-
degree of polymerization;
- k-ELISA:
-
kinetic-dependent enzyme-linked immunosorbent assay
- Mr :
-
relative molecular mass
- pI:
-
isoelectric point
- PS:
-
l-α-phosphatidylserine dipalmitoyl
- SDS-PAGE:
-
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
Anantharam, V., Patanjali, S.R., Surolia, A. (1985) A chitotetraose specific lectin from Luffa acutangola: Physicochemical properties and the assignment of orientation of sugars in the lectin binding site. Proc. Int. Symp. Biomol. Struct. Interactions, Suppl. J. Biosci. 8, 403–411
Boyd, W.C. (1963) The lectins, their present status. Vox Sang. 8, 1–32
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principe of protein-dye binding. Anal. Biochem. 72, 248–254
Broekaert, W.F., Pariss, J.V., Leyns, F., Joos, M., Peuhans, W.J. (1989) A chitin-binding lectin from stinging nettle rhizomes with antifungal properties. Science 245, 1100–1102
Conrath, U., Domard, A., Kauss, H. (1989) Chitosan-elicited synthesis of callose and of coumarin derivatives by parsley cell suspensions. Plant Cell Rep. 8, 152–154
Distler, J.J., Roseman, S. (1962) D-glucosamine oligosaccharides. Partial hydrolysis of chitosan. Methods Carbohydr. Chem. 1, 305–309
Domard, A. (1987) Determination of N-acetyl content in chitosan samples by CD measurements. Int. J. Biol. Macromol. 9, 333–336
Domard, A., Cartier, N. (1989) Glucosamine oligomers: preparation and characterization. Int. J. Biol. Macromol. 11, 297–302
Domard, A., Rinaudo, M. (1983) Preparation and characterization of fully deacetylated chitosan. Int. J. Biol. Macromol. 5, 49–52
Etzler, M.E. (1985) Plant lectins: molecular of biological aspects. Annu. Rev. Plant Physiol. 38, 209–234
Hadwiger, L.A., Beckman, J.H. (1980) Chitosan as a component of pea-Fusarium solani. Interactions. Plant Physiol. 66, 205–211
Hankins, C.H., Kindinger, J.I., Shannon, L.M. (1988) The lectins of Sophorajaponica III. Purification, properties and N-terminal amino acid sequences of five lectins from bark. Plant Physiol. 86, 67–70
Henrissat, B., Pérez, S., Tvaroska, I., Winter, W.T. (1987) The structures of cellulose. Characterization of the solid states. In: ACS Symposium Series, vol. 340, pp. 38–67, Atalla, R.H., ed. American Chemical Society, Washington
Heyraud, A., Rinaudo, M. (1978) Gel permeation chromatography of glucose oligomersthermodynamics and steric partition mechanisms. J. Chromatogr. 166, 149–158
Hustache, G., Mollard, A., Barnoud, F. (1975) Culture illimitée d'une souche anergiée de Rosa glauca par la technique des suspensions cellulaires. C.R. Acad. Sei. Paris D 281, 1381–1384
Kauss, H., Jeblick, W., Domard, A. (1989) The degrees of polymerization and N-acetylation of chitosan determine its ability to elicit callose formation in suspension cells and protoplasts of Catharanthus Roseus. Planta 178, 385–392
Laemmli, U.K. (1970) Cleavage of the structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Liénart, Y., Domard, A., Driguez, H. (1989) Chitosan as elicitor of β-d-glycanases from Rubus cells. In: Chitin and chitosan, pp. 225–231, Skjak-Braek, G., Anthonsen, T., Sandford, P. eds. Elsevier, London New York
Lis, H., Sharon, N. (1981) Lectins in higher plants. In: The Biochemistry of plants, pp. 372–447, Marcus, A., eds. Academic Press, New York
Miya, M., Iwamoto, R., Yoshikawa, G., Mima, S. (1980) IR spectroscopic determination of CONM content in highly deacetylated chitosan. Int. J. Biol. Macromol. 2, 323–324
Roberts, G.A.F., Domszy, J.G. (1982) Determination of the viscometric constants for chitosan. Int. J. Biol. Macromol. 4, 374–377
Roy, R., Katzenellenbogen, E., Jennings, H.J. (1983) Improved procedures for the conjugation of oligosaccharides to protein by reductive amination. Can. J. Biochem. Cell. Biol. 62, 270–275
Smith, H. (1978) Recognition and defence in plants. Nature 273, 266–268
Somogyi, M. (1952) Notes on sugar determination. J. Biol. Chem. 195, 19–23
Tsang, V.C.W., Wilson, B.C., Peralta, J.M. (1983) Quantitative, single-tube, kinetic-dependent enzyme-linked immunoabsorbent assay (k-ELISA). Methods Enzymol. 92, 391–413
Tsukada, S., Inoue, Y. (1981) Conformational properties of chitooligosaccharides: Titration, optical rotation, and Carbon-13 N.M.R. Studies of chito-oligosaccharides. Carbohydr. Res. 88, 19–38
Author information
Authors and Affiliations
Additional information
We are grateful to Professor Dr. H. Kauss (Fachbereich Biologie, Universität Kaiserslautern, FRG) for many helpful suggestions during the writing of this work. We also thank Dr. H. Driguez (C.E.R.M.A.V, University of Grenoble, France) for interesting discussions and Dr. D. Guest (University of Melbourne, Australia) for correcting the English manuscript.
Rights and permissions
About this article
Cite this article
Liénart, Y., Gautier, C. & Domard, A. Isolation from Rubus cell-suspension cultures of a lectin specific for glucosamine oligomers. Planta 184, 8–13 (1991). https://doi.org/10.1007/BF00208229
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00208229