Abstract
Background
Fingolimod is an oral sphingosine-1-phosphate–receptor modulator, which has demonstrated efficacy in clinical trials and has recently been approved for multiple sclerosis (MS) treatment in Kuwait. Post-marketing studies are important to demonstrate real-life efficacy and safety.
Objective
The objective of this study was to examine the efficacy and safety of fingolimod treatment in a clinical setting.
Methods
Using the national Kuwait MS registry, relapsing remitting MS patients who had been prescribed fingolimod for ≥6 months were retrospectively identified. Three-monthly clinical evaluations and 6-monthly magnetic resonance imagings (MRIs) were performed. Patient status pre- and post-treatment was compared using chi-square and Student t-tests.
Results
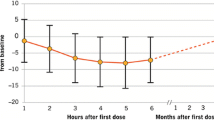
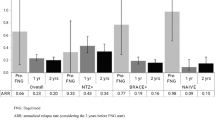
A total of 175 patients were included: 75.4 % female (n = 132); mean age 33.3 ± 9.2 years; mean disease duration 7.2 ± 5.2 years; mean fingolimod use 21.7 ± 9.1 months. Most had used previous disease-modifying therapy (78.9 %; n = 138), mainly interferons (66.9 %; n = 117). Twenty-three patients (11.4 %) discontinued/withdrew fingolimod; of whom eight had relapses. The proportion of relapse-free patients improved significantly (86.3 % vs. 32.6 %; p < 0.001), while the proportion of patients with MRI activity decreased (18.3.6 % vs. 77.7 %; p < 0.001). Mean expanded disability status scale (EDSS) score at the last visit improved when compared with pre-treatment (2.26 ± 1.49 vs. 2.60 ± 1.44; p = 0.03). Forty-three (24.6 %) patients experienced adverse events; headaches and lymphopenia were the most commonly reported adverse events.
Conclusion
Fingolimod treatment was associated with reduced relapse and MRI activity, and an improved EDSS score. Discontinuation/withdrawal rates and adverse events were low. Fingolimod presents a promising treatment for MS in Kuwait.
Similar content being viewed by others
References
Richards RG, Sampson FC, Beard SM, Tappenden P. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess. 2002;6(10):1–73.
Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61(11):1528–32.
Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain. 1989;112(Pt 6):1419–28.
Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis: the Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39(3):285–94.
Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352(9139):1498–504.
Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43(4):655–61.
Johnson KP, Brooks BR, Ford CC, Goodman A, Guarnaccia J, Lisak RP, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years: Copolymer 1 Multiple Sclerosis Study Group. Mult Scler. 2000;6(4):255–66.
Mehling M, Kappos L, Derfuss T. Fingolimod for multiple sclerosis: mechanism of action, clinical outcomes, and future directions. Curr Neurol Neurosci Rep. 2011;11(5):492–7.
Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401.
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–15.
Agashivala N, Wu N, Abouzaid S, Wu Y, Kim E, Boulanger L, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol. 2013;13:138.
Agashivala N, Wu N, Abouzaid S, et al. Comparison of compliance to fingolimod and other first-line disease modifying treatment among patients with multiple sclerosis. ACMP 2012 Education Conference, 3–5 October 2012, Cincinnati.
Tan H, Cai Q, Agarwal S, Stephenson JJ, Kamat S. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther. 2011;28(1):51–61.
Devonshire V, Lapierre Y, Macdonell R, Ramo-Tello C, Patti F, Fontoura P, et al. The global adherence project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18(1):69–77.
US Food and Drug Administration. In: Gilenya prescribing information; 2011. http://www.accessdata.fda.gov/drugsatfdadocs/label/2011/022527s002lbl.pdf.
Bergvall N, Makin C, Lahoz R, Agashivala N, Pradhan A, Capkun G, et al. Comparative effectiveness of fingolimod versus interferons or glatiramer acetate for relapse rates in multiple sclerosis: a retrospective US claims database analysis. Curr Med Res Opin. 2013;29(12):1647–56.
Havla J, Tackenberg B, Hellwig K, Meinl I, Krumbholz M, Seitz F, et al. Fingolimod reduces recurrence of disease activity after natalizumab withdrawal in multiple sclerosis. J Neurol. 2013;260(5):1382–7.
Alroughani R, Ahmed SF, Behbahani R, Khan R, Thussu A, Alexander KJ, et al. Increasing prevalence and incidence rates of multiple sclerosis in Kuwait. Mult Scler. 2014;20(5):543–7.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–52.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907–11.
http://c.ymcdn.com/sites/www.mscare.org/resource/collection/9C5F19B9-3489-48B0-A54B-623A1ECEE07B/mriprotocol2009.pdf. Accessed 21 Nov 2013.
Agius M, Meng X, Chin P, Grinspan A, Hashmonay R. Fingolimod therapy in early multiple sclerosis: an efficacy analysis of the TRANSFORMS and FREEDOMS studies by time since first symptom. CNS Neurosci Ther. 2014;20(5):446–51.
Cohen JA, Barkhof F, Comi G, Izquierdo G, Khatri B, Montalban X, et al. Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol. 2013;260(8):2023–32.
Ontaneda D, Hara-Cleaver C, Rudick RA, Cohen JA, Bermel RA. Early tolerability and safety of fingolimod in clinical practice. J Neurol Sci. 2012;323(1–2):167–72.
Maciejek Z, Wojcik-Draczkowska H, Wawrzyniak S, Niezgodzinska-Maciejek A. Evaluation of efficacy, safety and tolerability of fingolimod in patients with the relapsing form of multiple sclerosis: 12-month observation. A preliminary report. Neurol Neurochir Pol. 2013;47(2):145–51.
Ratchford JN, Costello K, Reich DS, Calabresi PA. Varicella-zoster virus encephalitis and vasculopathy in a patient treated with fingolimod. Neurology. 2012;79(19):2002–4.
Hanson KA, Agashivala N, Stringer SM, Balantac Z, Brandes DW. A cross-sectional survey of patient satisfaction and subjective experiences of treatment with fingolimod. Patient Prefer Adherence. 2013;7:309–18.
Yamout B, Alroughani R, Al-Jumah M, Khoury S, Abouzeid N, Dahdaleh M, et al. Consensus guidelines for the diagnosis and treatment of multiple sclerosis. Curr Med Res Opin. 2013;29(6):611–21.
Visser F, Wattjes MP, Pouwels PJ, Linssen WH, van Oosten BW. Tumefactive multiple sclerosis lesions under fingolimod treatment. Neurology. 2012;79(19):2000–3.
Pilz G, Harrer A, Wipfler P, Oppermann K, Sellner J, Fazekas F, Trinka E, Kraus J. Tumefactive MS lesions under fingolimod: a case report and literature review. Neurology. 2013;81(19):1654–8.
Nealon N. Severe multiple sclerosis relapse on fingolimod. Mult Scler. 2011;17:S53–276.
Castrop F, Kowarik MC, Albrecht H, et al. Severe multiple sclerosis relapse under fingolimod therapy: incident or coincidence? Neurology. 2012;78:928–30.
Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115(1):84–105.
Golden NH, Carlson JL. The pathophysiology of amenorrhea in the adolescent. Ann N Y Acad Sci. 2008;1135:163–78.
Acknowledgments
We thank the medical and administrative staff of the Dasman Diabetes Institute for their comprehensive logistic support in helping to establish the national MS registry.
Conflict of interest
Drs. Al-Hashel, Ahmed, and Behbehani have no conflicts of interest to declare. Dr. Alroughani has received speaker fees from Biologix, Novartis, GSK, Bayer, and Merck Serono. He has received travel support to attend scientific meetings from Novartis, GSK, Bayer, and Merck Serono, and has been a paid speaker for Biologix, Novartis, GSK, and Bayer.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
AL-Hashel, J., Ahmed, S.F., Behbehani, R. et al. Real-World Use of Fingolimod in Patients with Relapsing Remitting Multiple Sclerosis: A Retrospective Study Using the National Multiple Sclerosis Registry in Kuwait. CNS Drugs 28, 817–824 (2014). https://doi.org/10.1007/s40263-014-0185-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0185-z