Abstract
Carotid angioplasty and stenting (CAS) has emerged as a feasible and safe alternative for the management of extracranial carotid occlusive disease. The appropriate clinical setting, indications and techniques to maximize the benefit of this new approach are in constant evolution. The success of CAS relies not only on technique, device selection and management of complications but, maybe more importantly, on patient selection, peri-operative medical management and pre-procedural imaging and planning. The purpose of this review is to describe the peri-procedural and technical steps that can optimize the results of CAS.
Similar content being viewed by others
Introduction
Since the initial US Food and Drug Administration approval of the first interventional carotid artery stenting (CAS) device system for use in high-risk patients in 2004 [1], multiple trials have been conducted. The most recent of these is the multicenter randomized CREST trial, with its results published in July 2010 [2•].
The initial randomized trials and registries data not only confirmed the safety and efficacy of CAS, but also identified a set of high risk criteria describing cases in which an endovascular intervention might harbor higher risks for adverse events. The results of these trials have also confirmed carotid endarterectomy (CEA) as the current standard of care for most patients with carotid stenosis, mainly because of the uniformly reported lower rate of neurologic adverse events when compared to CAS. Among the objectives of this review, we attempt to identify high risk situations for CAS in order to address and optimize the peri-procedural factors that impact its results. This review will describe the pre-procedural workup, intra-procedural details and post-procedural factors that are known to impact outcomes of CAS.
Patient Selection
The indications for treatment for extracranial carotid occlusive disease are regarded to be the same whether CEA or CAS is contemplated. However, it is a constant matter of debate if the patient selection criteria for surgery can be applied to CAS. It is important to note that the benefit of CEA is based on its potential for decreasing the risk of stroke occurrence 3–5 years after the procedure [3]. In general, any condition that limits life expectancy will negate the benefit of revascularization of an asymptomatic carotid stenosis with either approach.
Patient selection for CEA or CAS may often be biased by provider or patient preferences. It is well accepted, nevertheless, that certain risk factors, such as prior neck irradiation, re-operative surgical fields and high lesions, may make CEA technically challenging and complicated, thus favoring CAS as a better option [4, 5]. In addition, congestive heart failure (III–V), multi-vessel coronary artery disease, LVEF <30 %, unstable angina, recent MI, renal failure on dialysis and liver failure have been described as physiologic variables conferring a high risk for complications after carotid endarterectomy [6]. CAS, therefore, may be desirable in this last patient population, keeping in mind that the benefit of revascularization for asymptomatic carotid disease in the setting of disabling medical comorbidities is questionable.
On the other hand, certain characteristics, which are described below, can make CAS a high risk procedure. A careful evaluation of the benefits and risks of either approach should be individualized to every patient. Proper selection of candidates for CAS is crucial to avoid complications and optimize outcomes.
Symptomatic Carotid Stenosis
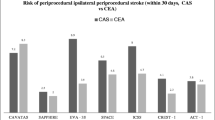
Results from several trials have consistently shown worse outcomes in symptomatic patients treated with CAS as compared to CEA. As early as 2004, subgroup analysis in the SAPPHIRE trial showed that among patients treated with CAS, a greater cumulative incidence of the primary neurologic endpoint was observed in the symptomatic patients (16.8 %) compared to the asymptomatic (9.9 %) [7]. In the EVA-3S, among symptomatic patients the incidence of any non-fatal strokes within 30 days was significantly higher in the CAS compared to the CEA group (8.8 vs. 2.7 %); this trial was terminated early for safety and futility reasons. The SPACE trial in 2006 also failed to show CAS non-inferiority when compared to CEA, showing a slightly higher rate of ipsilateral ischemic stroke and death at 30 days in symptomatic patients undergoing CAS [8]. The CAPTURE registry publication of predictors of outcomes in 2007 concluded that CAS is safe in high-risk patients with severe stenosis. However, when compared with the asymptomatic cohort, symptomatic patients treated with CAS had a sobering statistically significant higher risk of death or stroke (10.6 vs. 4.9 %) and death or major stroke (6.2 vs. 2.4 %) at 30 days. More recently, the CREST trial showed slightly higher but not significant incidence of stroke and death in the stenting group compared to the endarterectomy group among symptomatic patients (6.7 vs. 5.4 %; hazard ratio for stenting, 1.26; 95 % CI, 0.81 to 1.96) and an almost equal incidence of the primary endpoints among asymptomatic patients (3.5 vs. 3.6 %; hazard ratio, 1.02; 95 % CI, 0.55 to 1.86). These results suggest poorer outcomes for CAS in symptomatic patients compared to asymptomatic patients, thus advocating the cautious use of CAS in this patient population, primarily in the presence of other anatomic or medical high risk criteria. Symptomatic patients from a high grade carotid stenosis should be preferentially treated with CEA by current guidelines and recommendations [9•, 10•, 11].
Age
Of special importance is the subset of patients older than 80 years. Numerous studies have demonstrated that patients older than 75–80 years are perfectly suitable candidates for CEA [12, 13]. In addition, investigators in the recent CREST lead in trial results and other trials found increased rate of death and stroke in this group of patients (12 % in octogenarians vs. 3.23 % in non-octogenarians) after CAS. This observation has been repeated with such alarming regularity in other trials that age older than 80 years should be considered at least a relative contraindication to CAS [14, 15]. It should be noted, however, that increased risk with age actually starts at 75 years and accelerates beyond the age of 80 [5]. The etiology of the poor outcome in these patients remains to be elucidated. Factors such as arch elongation, arch calcification, and vessel tortuosity, seem to be more prevalent in the elderly and have been associated anecdotally with increased risk of CAS even in the younger patients. In addition, increased plaque thrombogenicity and vulnerability noted in elderly symptomatic patients may explain the increased rate of neurologic complications with CAS [16, 17].
Sex
Increased risk of stroke after CEA in women was first reported in the ACAS trial [18]. On the other hand ACST [19], NASCET [20] and CAVATAS demonstrated no difference by sex detected in the primary endpoint of the trials. Recently, according to a subgroup analysis of the CREST trial the peri-procedural risk of events seems to be higher in women who undergo CAS instead of CEA [21]. Peri-procedural events were observed in 31 (6.8 %) of 455 women assigned to carotid artery stenting compared with 16 (3.8 %) of 417 assigned to carotid endarterectomy (p = 0.064), whereas there was no significant difference among men. These results suggest a trend for higher peri-procedural stroke in women, and should be taken into account when choosing the treatment modality.
Anatomy
The number of intra-procedural micro emboli detected with trans-cranial Doppler scans [22] and the incidence of new embolic lesions detected by diffusion weighted MRI [23] show a higher incidence of embolic events in CAS when compared to patients undergoing CEA. This could be particularly important in the setting of adverse plaque characteristics. Long stenotic lesions (>15 mm) and involvement of the internal carotid ostium seem to be associated with increased incidence of peri-operative complications, especially when CAS is performed in elderly patients [24]. In addition, plaque characteristics have received some attention as a predictors of CAS outcomes, with some evidence that soft plaques with low echogenicity scores may be associated with significant neurologic adverse events [25]. Thus, anatomical characteristics should be considered in the treatment algorithm of carotid revascularization, recognizing the higher incidence of adverse events with long and ostial lesions, as well as vulnerable carotid plaques. Until further validation of these findings, caution should be exercised in stenting lesions in this particular subset of patients.
Medical Therapy
The most recent multi-society guidelines on the management of patients with extra-cranial carotid and vertebral artery disease (ECVD) describe the current keystones on the medical management of these patients [9•]. Medical therapy, regardless of any surgical or endovascular intervention, focuses on the specific management of hypertension, hyperlipidemia and diabetes. Smoking cessation is strongly recommended to reduce the risk of atherosclerosis progression and stroke. Furthermore, although no specific recommendations are made, the importance of obesity, lack of physical activity and the metabolic syndrome as potential risk factors for carotid disease and stroke is highlighted.
It is important to note that since the publication of trials comparing carotid revascularization to medical therapy with aspirin only, the non-operative intervention or medical management has been continuously refined and redefined. The current medical management of carotid occlusive disease (which entails the routine use of antiplatelet therapy, lipid lowering agents, angiotensin inhibitors and beta blockers) might have altered the natural history of the disease. This is supported by recent data suggesting a stroke risk of less than 1 % per year for asymptomatic carotid stenosis treated with best medical treatment (BMT) alone [26, 27].
Antiplatelet Therapy
Intimal injury during CAS releases pro-coagulants and exposes sub-endothelial platelet adhesive proteins, thus leading to rapid formation of thrombus and potential embolization. Most of the information on stent thrombosis is from extrapolation of the literature on the coronary bed [28, 29].
Aspirin (ASA) is an effective and safe antiplatelet agent, extensively investigated, and constitutes the mainstay of antithrombotic therapy. However, it shows marked inter-patient variability and some vasculopath patients appear to be resistant to its action [30]. Clopidogrel, an oral thienopyridine of the same family as ticlopidine but better tolerated, irreversively inhibits a chemoreceptor on platelet membranes, thus impairing adhesion. Dual therapy with clopidogrel and ASA has been able to demonstrate reduction in silent microemboli detected by TCD among patients with recent symptomatic carotid stenosis [31].
There is consensus among interventionalists to treat patients undergoing CAS in a similar fashion to those receiving coronary stents, thus a regimen consisting of ASA 81–325 mg/day and clopidogrel 75 mg/day to be started 4 days prior to the procedure is advocated. Alternatively a loading dose of 300 mg can be administered 4–6 h before the procedure followed by 75 mg daily [9•, 10•].
Regarding the duration of the antiplatelet therapy, most practitioners recommend 4 weeks of therapy to prevent acute and sub-acute thrombosis, documented to occur up to 30 days after stenting [32].
Over the last few years special attention has been devoted to the reported “resistance” to antiplatelet therapies in specific patients. “Resistance” has been described as the occurrence of occlusive cardiovascular disease events despite regular intake of these agents at recommended doses [33]. The frequency of this phenomenon has been reported to range from 1 to 45 % for aspirin and/or clopidogrel [34]. The mechanisms or resistance are poorly understood and multifactorial, thus despite the variety of tests available, no consensus exists regarding the reference standard for measuring platelet activation [35]. Several explanations can account for Clopidogrel resistance or treatment failure, including non-compliance, variable platelet response, genetic variability of cytochrome P450 enzymes, and interaction with several classes of medications (statins, proton pump inhibitors, calcium channel blockers). The failure of antiplatelet therapy will likely result in an adverse clinical outcome, however, there is no clear strategy for the management of this problem, an initial approach would be to correct clinical factors that may cause therapeutic resistance. Physicians must ensure proper patient compliance while also minimizing drug–drug interactions. Although testing for Clopidogrel resistance is not routinely recommended, given the current evidence, it should be selectively entertained in patients suspected of having recurrent failure of antiplatelet therapy prior to CAS. In addition, optimal control of glucose levels and cholesterol levels can reduce platelet reactivity and should be actively pursued.
Antihypertensive Therapy
Hypertension increases the risk of stroke. For each 10-mmHg increase in blood pressure, the risk of stroke increases by 30 to 45 % [36]. A meta-analysis of more than 40 trials and 188,000 patients found a 33 % decreased risk of stroke for each 10-mmHg reduction in systolic blood pressure [37]. A large body of literature is available to support the reduction of recurrent strokes by antihypertensive therapy regardless of the agent used [38]. In patients who had experienced ischemic stroke, administration of a combination of angiotensin converting enzyme inhibitors and a diuretic significantly reduced the risk of recurrent ischemic events compared with placebo [39].
Patients with ischemic stroke or TIA, regardless of the source, should receive anti-hypertensive treatment beyond the hyper-acute period [26]. According to the American Heart Association guidelines for the management of extracranial carotid disease, antihypertensive treatment is probably indicated in patients with hypertension and symptomatic extracranial carotid or vertebral atherosclerosis. However, the benefit of treatment to a specific target blood pressure (e.g., below 140/90 mmHg) has not been established in relation to the risk of exacerbating cerebral ischemia [9•, 10•].
Of note, symptomatic patients with severe carotid artery stenosis represent a challenging situation since it is not known whether antihypertensive therapy is beneficial or harmful by reducing cerebral perfusion. The Seventh Report of the Joint National Committee for the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) offers no specific recommendation for treatment of hypertension in patients with severe ECVD [40].
It is instrumental to establish a patient targeted antihypertensive regimen to control blood pressure before and after CAS in order to maximize its outcomes [9•, 10•].
Statins
Statins belong to a class of drugs known to inhibit 3-hydroxy 3-methylglutaryl coenzyme A reductase, and block hepatic cholesterol synthesis. They became the gold standard for treatment of hypercholesterolemia since their FDA approval in 1987. They have proven to successfully reduce the incidence of stroke among patients at increased risk for cardiovascular disease; the Stroke Prevention Aggressive Reduction of Cholesterol Levels (SPARCL) [27] showed that the use of atorvastatin reduced the overall incidence of stroke and of cardiovascular events. Furthermore, statin use was independently associated with a 75 % reduction in the odds of death and 45 % reduction in the odds of ischemic stroke or death among patients with symptomatic carotid disease. There are data indicating that statin use may reduce long-term incidence of restenosis following CEA [41] and a similar benefit of reducing neurologic morbidity among patients undergoing carotid angioplasty and stent procedures has been documented [42]. The precise mechanism underlying the “pleiotropic” favorable effects of the drug on platelet adhesion, thrombosis, plaque stability, and inflammation is unclear; however, statins given peri-operatively may not only lower lipid levels but might stabilize the arterial wall as well. The use of statins (pravastatin, simvastatin, lovastatin or cervistatin) for at least 1 week prior to the procedure has been shown to reduce the incidence of stroke, myocardial infarction, and death within 30 days after CAS [43]. Magnetic resonance imaging (MRI) using ultra-small super-paramagnetic iron oxide (USPIO) particles has been shown to identify inflammatory changes by monitoring macrophage uptake, a major component of high-risk (vulnerable) plaques [44]. The ATHEROMA trial (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) used this new MRI technology to compare the effects of a low (10 mg) vs. a high (80 mg) atorvastatin daily dose in the carotid plaque. It concluded that aggressive lipid-lowering therapy over a 3-month period is associated with significant reduction in USPIO-defined inflammation [45]. The use of aggressive statin therapy is therefore likely to improve the outcomes of CAS by reducing adverse neurologic and myocardial events through different mechanistic effects.
Imaging
Determination of degree of carotid artery stenosis is of critical importance when deciding whether a patient warrants intervention. Physicians rely heavily in diagnostic imaging to make this decision. The fast evolution pace of current non-invasive imaging, as well as their continuously increasing accuracy and resolution has relegated purely diagnostic cerebral angiography to a second plane.
Plaque characterization in US and also in CTA and MRA may have special relevance in the era of carotid stenting, since identification of a high-risk vulnerable plaque may be useful when deciding whether to perform a stent procedure or an endarterectomy.
The near future might bring the ability to create a computer model and perform a simulated procedure to test the operator, the devices and to prove the feasibility of CAS for a given patient [46, 47].
Ultrasound
Currently, physicians most commonly rely on Duplex ultrasound when planning an operative intervention for carotid stenosis. The current guidelines advocate for the use of US in the initial evaluation of any patient that presents with acute, focal ischemic neurological symptoms corresponding to the territory supplied by the carotid arteries. Ultrasound is a non-invasive and relatively inexpensive diagnostic method capable of detecting and quantifying the degree of stenosis based on velocity measurement and Doppler waveform analysis. B-mode imaging is also able to evaluate specific additional characteristics of the carotid plaque that might make it high risk for embolization: a hyperechoic, echodense or bright plaque is indicative of an indurated, relatively stable, calcified plaque. On the other hand a hypoechoic, echolucent or dark plaque is indicative of blood or lipid rich plaque, which is likely prone to embolize during CAS [48]. Ultrasound, however, is operator dependent, is limited in highly calcified lesions and the criteria for grading stenosis may differ among different institutions. Furthermore, CAS requires full understanding and precise knowledge of the specific anatomy of the intra- and extra-cranial vascular tree, and current generation US does not provide an adequate and reliable road-map to help in device selection and plan a detailed intervention.
CTA and MRA
Computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) have gained a prominent role in CAS planning in recent years. Detailed imaging and understanding of the anatomy and quality of the aortic arch, neck and cerebral vasculature is necessary to be aware of potential difficulties during the procedure such as tortuosity of the vessels. Severe arch atherosclerosis (eggshell aorta), arch irregularities (shaggy aorta), diffuse common carotid artery disease, eccentric stenoses, severe angulations of the bifurcation and kinking or disease of the distal internal carotid artery should raise a red flag, since they might represent relative contraindications for an endovascular procedure. CT and MR imaging also provide documentation of pre-procedural pathologic changes in the brain and serve as a platform for measuring and sizing the arteries in order to choose the most appropriate devices to use during the intervention.
The current American guidelines recommend the use of magnetic resonance angiography (MRA) or computed tomography angiography (CTA) in patients with acute, focal ischemic neurological symptoms when sonography either cannot be obtained or yields equivocal or otherwise non-diagnostic results. In general and in asymptomatic patients, when initial noninvasive images are inconclusive, additional examination by use of another imaging method is reasonable.
Magnetic resonance angiography without contrast is reasonable for assessing the extent of disease in patients with symptomatic carotid atherosclerosis and renal insufficiency or extensive vascular calcification. CTA, on the other hand, is reasonable for evaluation of patients with clinically suspected significant carotid atherosclerosis who are not suitable candidates for MRA because of claustrophobia, implanted pacemakers, or other incompatible devices [9•].
The use of axial imaging before CAS can therefore help improve CAS outcomes by providing an anatomic roadmap and by allowing the operator to anticipate high risk anatomic situations and procedural events.
Catheter Based Contrast Angiography
Conventional angiography was once considered the standard against which other methods of vascular imaging were compared; however, its use as a screening modality has been abandoned due to its high risk and cost. Currently, its use is of particular importance in situations where adequate delineation of the disease cannot be obtained by other methods or when imaging studies have yielded discordant results [10•].
The most feared complication is stroke, initially reported as high as 1.2 % in the ACAS trial [49], however the incidence of which should be <1 % when performed by experienced physicians, according to more recent trials [50–52].
The selective utilization of angiography to rule out significant disease is rarely indicated, thus the operator should ideally be prepared to perform an intervention if needed.
Intravascular Ultrasound (IVUS)
Intravascular ultrasound technology, which has been gaining acceptance in the coronary and peripheral arterial fields, allows the operator to gather intra-procedural detailed information about the diameter of the vascular lumen, extent of atherosclerosis, and degree of calcification. It is also used to evaluate the outcome of an intervention (i.e., detect arterial dissection after angioplasty and plaque and stent configuration after deployment) [53, 54].
The incorporation of IVUS during CAS has been addressed in a few studies and has focused mainly on the safety of the technique and its potential contribution to the success of carotid revascularization [55].
Although there is some evidence showing that incomplete stent expansion or small post-procedural stent diameter may be associated with a greater risk of restenosis [56], there are limited data regarding the impact that IVUS completion imaging might have in preventing re-stenosis, reducing strokes or improving overall outcomes.
Although used safely in small series of patients, more evidence is needed before the incremental risk associated with the additional catheter manipulation required to traverse stenotic lesions can be justified.
Device Selection
Selection of the appropriate equipment is of paramount importance. Devices able to negotiate the aortic arch and carotid system tortuosity with adequate support and minimal trauma are ideal. The operator should be familiar with a set of devices for both routine and complex CAS, and should use them on a regular basis to achieve proficiency. Adopting routine CAS steps and familiarity of the operator and staff with a set of devices and tools ensure the smooth flow of the procedure.
Sheaths and Guiding Catheters
The anticipated stent size required to perform the procedure should be placed at the time of initial access, usually a 6 or 7 Fr sheath. Following femoral access, systemic anticoagulation is indicated prior to device manipulation in the aortic arch. This can help minimize thromboembolic complications.
Selecting Catheters
Multiple selective catheters are available. These are generally available in 100–125 cm length. The selective catheters can be grouped in two major categories:
Simple curve catheters have a primary angled tip. Such catheters are placed in the arch proximal to the ostium of the target artery and rotated toward the orifice as the catheter is being withdrawn. Among these catheters are the angled-taper Glidecath, the H1 headhunter, The JB 1 and the vertebral catheter (Angiodynamics, Latham, NY).
Complex curve catheters have, in addition to a primary angle tip, a secondary curve and must be reshaped in the aorta to their original configuration to achieve cannulation. The two or more curves are useful in complex arterial configuration. The catheter head is positioned near the branch vessel and advanced or withdrawn to select the target vessel. Among these catheters we have the H3 headhunter, the Simmons 1, 2 and 3, JB 2 and the VTK catheters (Angiodynamics, Latham, NY).
Guidewires
Placement of the sheath or the guiding catheter into the CCA requires a relatively stiff 0.035-inch 260 cm long wire. The wire is advanced into the ECA in order to obtain enough purchase to advance the sheath or guiding catheter into the CCA. This allows adequate guidewire length placed beyond the carotid bifurcation for the subsequent placement of the carotid sheath. Passage of the stiff exchange guidewire into the small external carotid artery branches must be performed with caution to avoid injury or perforation to these small branches, especially avoiding the lingual and ophthalmic branches. To minimize thromboembolic complications, the target lesion is not manipulated at this stage of the procedure.
Embolic Protection Devices (EPD)
Pharmacologic strategies to minimize platelet aggregation and thrombus formation are not sufficient to prevent cerebral embolization during CAS. Previous studies have demonstrated the embolization of clot and plaque components during carotid manipulation [57, 58]. Two types of cerebral protection strategies are currently widely used: distal filtration and reversal of flow.
Distal filtration is the most widely used type of cerebral protection, its principle being to trap particulate debris while maintaining blood flow during the procedure. Most of the devices are calibrated to filter particles >100 u (70–140u). Filters are typically mounted on a 0.014 wire in a monorail or over the wire (OTW) configuration and are recaptured after CAS with a dedicated retrieval system. The AcuNet filter (Abbott, Illinois) was the first of these to obtain FDA approval. The Spider RX (ev3 Inc., Minneapolis, MN), the AngioGuard XP (Cordis, Warren, NJ), the FilterWire EZ (Boston Scientic, Natic, MA), the EmboShield Pro (Abbott, Illinois) are some of the filters also available in the USA.
The disadvantages of the filters are similar to previously utilized cerebral protection balloons; they cross the lesion without protection and pose the potential risk of arterial injury during deployment. In addition, suboptimal apposition can cause distal embolization and the filter itself can fill with debris due to its limited volumetric capacity [59, 60]. However, the main advantage of these devices is their ability to perform the procedure without interruption of cerebral blood flow.
Flow reversal (proximal occlusion) devices, on the other hand, can provide cerebral protection prior to crossing the target lesion. A reversal of flow in the ICA is achieved by occlusion of the CCA and ECA by a compliant balloon and either active syringe aspiration (Mo.Ma system, Invatec, Italy) through a side arm [61] or by continuous arterio-venous shunt from the ICA to the femoral vein (Gore Neuro protection system or Parodi anti-embolic device, Gore & Associates, Flagstaff, AZ) [62]. These devices could be useful for the treatment of friable and tight lesions in tortuous arteries where placement of filters or balloons might prove to be difficult or risky. Proximal occlusion devices require larger introducers and are technically complex. Intolerance to flow blockage is also encountered in 5.7–7.6 % of the patients [63] and could be dangerous in those with contralateral carotid occlusion. Nevertheless, this type of cerebral protection has been reported to be safe and effective in recent series, with a low rate of procedural adverse events [64]. Recent studies suggest that the differential in adverse neurologic event rates in CAS between symptomatic and asymptomatic patients may be ameliorated by the use of flow reversal embolic protection strategies in symptomatic patients.
Flow reversal with transcervical access has been recently described as minimizing the neurologic complications of CAS. It eliminates some of the complications associated with the transfemoral route, which include the hazards of wire and catheter manipulation in the aortic arch. In this setting, Criado et al described in 2004 a technique for transcervical CAS with flow reversal protection [65]. Although the experience is still small and prospective trials are needed, the short-term and long-term outcomes are also good and appear comparable with the reported results for carotid revascularization by endarterectomy [66, 67]. This technique promises to improve the neurologic outcomes of CAS as it has been associated with a lower incidence of new ischemic brain infarcts on diffusion-perfusion magnetic resonance imaging when compared to transfemoral CAS with a distal filter [68].
Angioplasty Balloons and Stents
Multiple low profile balloons are available for carotid angioplasty. A rapid exchange (monorail) system is instrumental and mandatory, as it minimizes manipulation across the lesion. The diameters range from 2 and 4 mm for pre-dilatation purposes to 4–6 for mm for post-stenting dilatation. After the lesion has been crossed and the filter deployed, it is common to pre-dilate the lesion with a 3 or 4 mm rapid exchange balloon. Some operators routinely administer small doses of atropine (0.25–0.5 mg) before balloon dilatation, except in patients with a recurrent stenosis.
Post-stenting balloon angioplasty should be focal and judiciously utilized to gently mold the stent at the site of lesion, which allows for future remodeling. Aggressive large balloon angioplasty should be avoided to minimize thromboembolic complications. The stent provides continuous expansible energy after the procedure, and the urge to disrupt the lesion more than necessary should be avoided. The balloon should also be maintained within the stent to avoid dissection during post-dilatation.
All carotid stents are self-expandable and they are all constructed from nitinol (nickel-titanium alloy) except the Wallstent (Boston Scientific, Natik, MA), which is a braided-mesh frame constructed from stainless steel (a cobalt alloy). There are 3 types of carotid stent designs: open cell, closed cell and hybrid.
Open cell stent designs have a bigger free cell area and lighter scaffolding, which allows some individual segments of the stent to move more freely. Open-cell stents are able to conform to more tortuous anatomy without significant kinking. On the other hand, they might show decreased ability to trap plaque and thrombus. The Precise stent (Cordis Endovascular Inc, Warren, NJ), Acculink stent (Abbott, Abbott Park, IL) and the Protegé (ev3 Inc. Minneapolis, MN) are examples of FDA approved open cell stents.
Closed-cell stents have a smaller free cell area and denser scaffolding, providing greater radial force, greater rigidity, and potentially improved ability to trap plaque or thrombus between the outside of the stent and the vessel wall. The higher rigidity of these stents, however, might make them prone to fracture and also more likely to deform the vessel in which they are placed, creating kinking of the vessel and poor wall apposition. The Xact carotid stent (Guidant/Abbott Vascular) and the Carotid Wallstent Monorail (Boston Scientific, Natik, MA) are among these kind of stents.
Recently, a hybrid stent, the Cristallo Ideale stent (Invatec/Medtronic, Roncadelle, ITA), became available for use in Europe. This stent has been designed with an open cell configuration in the proximal and distal ends to maximize conformability, and closed cell configuration in the middle portion of the stent to prevent plaque prolapse through the stent struts. This stent platform seems to be safe and effective in the peri-procedural period [69].
Stent tapering is advantageous when the common carotid artery (CCA) and internal carotid artery (ICA) differ significantly in size. There is evidence of decrease in the rate of re-stenosis with the use of tapered stents in this situation [70]. The Wallstent self-tapers, however, and because of its woven design, it substantially foreshortens with larger diameters. The Precise stent also tolerates mismatch in size owing to a relatively small number of connecting bridges. Specifically designed tapered stents include the Acculink Xact (Abbott, Abbott Park, IL) and the Protegé (ev3 Inc. Minneapolis, MN).
Given the differences in stent design and behavior, stent choice should be carefully tailored to the carotid anatomy and the plaque characteristics. Adequate sizing of the device is of utmost importance and should be guided by pre- and intra-procedural measurements, aiming for slight oversizing and complete lesion coverage, typically spanning the carotid bifurcation.
Intraprocedural Technical Considerations
In patients requiring an initial arch arteriogram, a 30°–45° left anterior oblique (LAO) projection generally provides the best visualization (“open up”) of the brachio-cephalic arteries’ take off. Of note, the best projection for evaluating the right innominate bifurcation is the right anterior oblique (RAO). When diffuse or shaggy disease is observed at the level of the aortic arch, compromising the take off of the brachiocephalic arteries, selective catheterization should be cautiously done or even avoided.
Heparin to achieve ACT >250 s should be given prior to selective catheterization of the aortic branches. Careful monitoring of ACT is recommended to avoid excessive anticoagulation. Bivalrudin has also been proven to be a safe and efficient anticoagulation strategy for CAS and could be considered as an alternative to heparin [71, 72].
During catheter exchange and manipulation, extra care should be taken to avoid potential embolization of debris, thrombus and air bubbles. Use of a manifold system is helpful in that regard.
Once the ostium of the common carotid artery has been negotiated, it is not recommended to advance the wire to the ICA, but rather to park it in the CCA or the ECA; the wire should provide enough purchase to avoid loss of access from the CCA while advancing the catheter. The operator should also avoid tension accumulation while advancing the catheter, since this might cause a sudden jump of the wire-catheter complex and inadvertent forward movement into the bifurcation or the ICA.
Once the catheter is placed at the level of the common carotid artery, an angiogram to visualize the carotid bifurcation, as well as the cerebral vessels in the Townes and lateral projection, is often obtained. Detailed and specific knowledge of the brain vascularization is instrumental in identifying and delineating collateral pathways and the pre-interventional cerebral perfusion configuration.
Baro-reflex responses such as bradycardia, hypotension, and vasovagal reactions occur in 5–10 % of cases, but have been reported in as many as 33 % of patients undergoing CAS [73, 74]. Most are transient and do not require ongoing treatment after the procedure. Asystole during balloon inflation is transient and responds to balloon deflation. Pre-treatment with atropine or glycopirrolate prior to angioplasty is often used to prevent bradycardia or asystole during carotid angioplasty and stent placement [75, 76]. The operator should be aware that patients on pre-operative beta-blockers might not respond well to atropine.
Complications and Bailout Techniques
Embolism
Cerebral embolization might result from the dislodging of an organized clot from the surface of the plaque, debris, plaque fragments and a mixture of fibrin, cholesterol clefts, red and white cell aggregates [57].
The prevention of micro-embolization depends on optimization of pharmacological treatment outlined above and on gentle technique.
The primary role of the EPDs is the capture of macro-emboli. Unfortunately, embolism still occurs, either due to insufficient EPD seal, distal embolization caused by the deployment of the EPD itself, dislodged thrombus prior to proper placement of the device or simply by the lack of its use. Embolization can also occur after the completion of the procedure through the stent struts. Acute emboli lodging in the middle cerebral artery can be fragmented using balloon angioplasty or by guide wire manipulation under the risk of further distal embolization [77]; this could be particularly helpful in cases where the embolic material is plaque-like, and not effectively dissolved by thrombolytic agents.
Recombinant TPA are commonly used thrombolytic agents. These are delivered through super-selectively placed micro-catheters. Recombinant TPA may be given as a 5-mg bolus, followed by slow-infusion (maximum dose 20 mg). Control angiography at regular intervals (e.g. every 15 min) should be performed and continued for 1 h or until recanalization has been achieved [78]. Other techniques for neurorescue involve the use of intra-cerebral clot retrieval devices such as the Merci (Stryker; Kalamazoo, MI, USA) or the Solitaire (Covidien/ev3; Dublin, Ireland). If the operator is not familiar with neurorescue techniques and maneuvers, it is imperative to identify someone at the same institution who is, in the rare event such interventions are needed.
Thrombosis
Acute in-stent thrombosis in CAS is a potentially fatal complication. With a rare incidence of 0.5–2 % [79], this life threatening complication seems to be related to the lack of treatment with combination antiplatelet therapy [80]. Treatment consists of intra-arterial thrombolysis at dose regimens described above for embolism. Treatment with intra-carotid administration of 0.25 mg/kg of abciximab intra-arterially, followed by a continuous intravenous infusion (9 μg/min for 12 h), has also been reported [81].
Thrombosis occurring during the procedure itself seems to be associated with the use of EPD [82], likely due to dislodgement and accumulation of debris and thrombus in the device. There are reports of thrombosis arising on the wire of the filter system [83]. Treatment consists of local administration of abciximab or aspiration of thrombus followed by retrieval of the filter device using the guiding catheter or sheath already in place.
Spasm
Spasm can occur during any procedure in the carotid artery. It seems to occur more frequently in cases where embolic protection devices are used [84]. Treatment is necessary in cases of flow-limiting spasm, and consists of the local administration of vasodilators, either nitroglycerin or papaverin [85]. In cases where the embolic protection device seems to be the cause of the spasm, advancement of the device may be useful.
Dissection
Dissection is a rare complication during carotid artery stenting. If flow reduction is not hemodynamically significant, expectant management could be advocated; however, if the flow impairment is judged to be significant, treatment consists of either insertion of a second stent, or urgent surgical repair [86].
Post-operative Care and Follow-up after CAS
Patients are monitored in the hospital overnight. It is not uncommon for patients to respond to carotid sinus distension with bradycardia and hypotension [76]. Hypotension may last from hours to days, depending on the sensitivity of the baroreceptors. Occasionally, 24–48 h of inotropic support is required before the carotid sinus adapts to the radial force of the self-expanding stents. The presence of significant hypotension in the absence of bradycardia is unusual in the immediate post procedure period; it is worth emphasizing that other causes (e.g. retroperitoneal bleed, myocardial infarction) should also be excluded.
In hypertensive patients and in patients with critical stenosis, a transient post-procedural confusional state with headaches and transient localized symptoms not associated with angiographic changes has been reported by Vitek et al. CT scan imaging will demonstrate mild hemispheric swelling and effacement of sulci, suggesting adequate or improved perfusion. These symptoms resolve over 24 h with good control of blood pressure [87].
Cerebral hyperperfusion syndrome (HPS), is a potentially life threatening neurological syndrome characterized by the triad of: (1) unilateral headache, (2) seizures and (3) focal neurological deficit. In its extreme form it can present as intracerebral hemorrhage. Originally, HPS was described in patients undergoing carotid endarterectomy for severe carotid stenosis; more recently however, it is a well documented complication of CAS. It is believed to result from hyperperfusion of blood into an unprotected or damaged brain parenchyma, resulting in maximal dilatation of intra-parenchymal arterioles and impaired cerebral autoregulation. The incidence of HPS after CEA has been reported to be around 3 and 7 % after carotid stenting [88]. This is particularly prominent in patients with severe stenosis and poor cerebro-vascular reserve [89]. Post-procedurally, tight blood pressure control is mandatory and the systolic blood pressure should be maintained between 100 and 140 mmHg. A combination of short acting IV beta-blockers and nitroglycerine is the initial therapy to control severe hypertension. HPS occurs mainly within the first 2 weeks after CAS and may be triggered by poorly controlled hypertension. In current practice, patients typically are discharged home the day after the procedure, so it is important to be conscious of this complication since it can occur in the outpatient setting. Continuous blood pressure monitoring after discharge is important since the patient’s anti-hypertensive medications might have been stopped in the hospital after a transient reduction in blood pressure post-procedurally.
Prior to discharge, NIH stroke scale classification should be recorded. Follow-up includes 1 month, 6 month, and yearly clinical evaluation and duplex examination.
Conclusions
Carotid artery stenting is an effective treatment for carotid stenosis in the appropriate setting and for the appropriate patient population.
Patient selection is the cornerstone to secure optimal outcomes, it requires thorough imaging, detailed diagnostic evaluation and a cautious decision making. Patients at high risk for stroke with CAS such as symptomatic patients and octogenarians should be treated only selectively with CAS to minimize the risk of neurologic adverse events.
Peri-procedural medical management is of utmost importance to optimize the results of CAS. Blood pressure control, hyperlipidemia management and adequate antiplatelet therapy are mandatory. In addition, overall management of comorbidities such as diabetes, concurrent CAD and pulmonary issues by a multispecialty team is also necessary.
Optimal results will likely derive from putting institutional protocols in place that allow careful patient selection, best medical management, and integration of a highly skilled interventional team.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
S Food and Drug Administration Centre for Devices and Radiological Health Medical Devices Advisory Committee Circulatory System Devices Panel Meeting. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108347.htm. Accessed 20 Oct 2012.
• Brott TG, Hobson RW II, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010;363:11–23. Multicenter, prospective randomized trial comparing CEA and CAS. This trial has been a source of multiple interpretations. It is important reference point to comprehend the current understanding of CAS.
North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–53.
Veith FJ, Amor M, Ohki T, et al. Current status of carotid bifurcation angioplasty and stenting based on a consensus of opinion leaders. J Vasc Surg. 2001;33:S111–6.
Mansour MA, Kang SS, Baker WH, et al. Carotid endarterectomy for recurrent stenosis. J Vasc Surg. 1997;25:877–83.
Celis R, Chaer RA. Carotid angioplasty and stenting: evolution and current status. Hosp Pract. 2011;39:62–70.
Bowens NM, Fairman RM. Carotid artery stenting: clinical trails and registry data. Semin Vasc Surg. 2010;23:148–55.
Ringleb PA, Allenberg J, Brückmann H, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–47.
• Thomas G. Brott, Jonathan L. Halperin, Suhny Abbara, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124(4):489–532. As the most recent American multi-society guideline for the management of patients with extracranial carotid artery and vertebral disease, these recommendations represent the latest consensus among specialties treating carotid disease as well as the backbone of treatment strategies offered and accepted in the United States.
• Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK; Society for Vascular Surgery. Updated society for vascular surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1–e31. These guidelines published by the Society for Vascular Surgery after the release of the multispecialty guidelines include recommendations based on a meta-analysis of 13 trials and nearly 7500 patients and clarifies the position of a specialty accredited to perform both CEA and CAS.
Liapis CD, Bell PR, Mikhailidis D, Sivenius J, Nicolaides A, Fernandes e Fernandes J, Biasi G, Norgren L, ESVS Guidelines Collaborators. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg. 2009;37:1–19.
Schneider JR, Droste JS, Schindler N, et al. Carotid endarterectomy in octogenarians: comparison with patient characteristics and outcomes in younger patients. J Vasc Surg. 2000;31:927–35.
Rockman CB, Jacobowitz GR, Adelman MA, et al. The benefits of carotid endarterectomy in the octogenarian. Ann Vasc Surg. 2003;17:9–14.
Stanziale SF, Marone LK, Boules TN, et al. Carotid artery stenting in octogenarians is associated with increased adverse outcomes. J Vasc Surg. 2006;43:297–304.
Voeks JH, Howard G, Roubin GS, Malas MB, Cohen DJ, Sternbergh WC 3rd, Aronow HD, Eskandari MK, Sheffet AJ, Lal BK, Meschia JF, Brott TG, CREST Investigators. Age and outcomes after carotid stenting and endarterectomy: the carotid revascularization endarterectomy versus stenting trial. Stroke. 2011;42:3484–90.
Aronow HD, Shishehbor M, Davis DA, Katzan IL, Bhatt DL, Bajzer CT, Abou-Chebl A, Derk KW, Whitlow PL, Yadav JS. Leukocyte count predicts microembolic Doppler signals during carotid stenting: a link between inflammation and embolization. Stroke. 2005;36:1910–4.
Kelly P, Bhatt DL. Identification of vulnerable plaque: the quest continues. J Invasive Cardiol. 2007;19:55–7.
Executive Committee for the Asymptomatic Carotid Atherosclerosis Study: endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428
Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet. 2004;363:1491–502.
Barnett HJM, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. 1998;339:1415–25.
Howard VJ, Lutsep HL, Mackey A, Demaerschalk BM, Sam AD II, Gonzales NR, Sheffet AJ, Voeks JH, Meschia JF, Brott TG, CREST investigators. Influence of sex on outcomes of stenting versus endarterectomy: a subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Lancet Neurol. 2011;10(6):530–7.
Crawley F, Clifton A, Buckenham T, Loosemore T, Taylor RS, Brown MM. Comparison of hemodynamic cerebral ischemia and embolic signals detected during carotid endarterectomy and carotid angioplasty. Stroke. 1997;28:2460–4.
Schnaudigel S, Gröschel K, Pilgram SM, Kastrup A. New brain lesions after carotid stenting versus carotid endarterectomy: a systematic review of the literature. Stroke. 2008;39:1911–9.
Sayeed S, Stanziale SF, Wholey MH, Makaroun MS. Angiographic lesion characteristics can predict adverse outcomes after carotid artery stenting. J Vasc Surg. 2008;47:81–7.
Biasi GM, Froio A, Diethrich EB, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation. 2004;110:756–62.
Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: cosponsored by the Council on Cardiovascular Radiology and Intervention. Stroke. 2006;37:577–617.
Amarenco P, Bogousslavsky J, Callahan A, et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59.
Hirsh J, Bhatt DL. Comparative benefits of clopidogrel and aspirin in high-risk patient populations: lessons from the CAPRIE and CURE studies. Arch Intern Med. 2004;164:2106–10.
Keller TT, Squizzato A, Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease. Cochrane Database Syst Rev. 2007;3:CD005158.
Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke. 1994;25:2331–6.
Dittrich R, Ritter MA, Kaps M, et al. The use of embolic signal detection in multicenter trials to evaluate antiplatelet efficacy: signal analysis and quality control mechanisms in the CARESS (clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis) trial. Stroke. 2006;37:1065–9.
Cremonesi A, Manetti R, Setacci F, et al. Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke. 2003;34:1936–41.
Wang TH, Bhatt DL, Topol EJ. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur Heart J. 2006;27(6):647.
Angiolillo DJ. Variability in responsiveness to oral antiplatelet therapy. Am J Cardiol. 2009;103(3):27A.
Michos ED, Ardehali R, Blumenthal RS, Lange RA, Ardehali H. Aspirin and clopidogrel resistance. Mayo Clin Proc. 2006;81(4):518–26.
Cruickshank JM, Fox K, Collins P, Alderman MH, Kaplan NM, Jenkinson ML. Meta-analysis of hypertension treatment trials. Lancet. 1990;335:1092–4.
Lawes CM, Bennett DA, Feigin VL, et al. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35:776–85.
Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741–8.
PROGRESS Collaborative Group. Randomised trial of a perindopril based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–41.
Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–72.
Perler BA. The effect of statin medications on perioperative and long-term outcomes following carotid endarterectomy or stenting. Semin Vasc Surg. 2007;20:252–8.
Verzini F, De Rango P, Parlani G, Giordano G, Caso V, Cieri E, Isernia G, Cao P. Effects of statins on early and late results of carotid stenting. J Vasc Surg. 2011;53(1):71–9.
Gröschel K, Ernemann U, Schulz JB, Nägele T, Terborg C, Kastrup A. Statin therapy at carotid angioplasty and stent placement: effect on procedure-related stroke, myocardial infarction, and death. Radiology. 2006;240:145–51.
Trivedi RA, Mallawarachi C, U-King-Im JM, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–6.
Tang TY, Howarth SPS, Miller SR, et al. The ATHEROMA (atorvastatin therapy: effects on reduction of macrophage activity) study: evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–50.
Chaer RA, DeRubertis BG, Lin SC, et al. Simulation improves resident performance in catheter-based intervention results of a randomized controlled study. Ann Surg. 2006;244:343–52.
Auricchio F, Conti M, De Beule M, De Santis G, Verhegghe B. Carotid artery stenting simulation: From patient-specific images to finite element analysis. Med Eng Phys. 2011;33:281–9.
Tedesco MM, Lee JT, Dalman RL, et al. Postprocedural microembolic events following carotid surgery and carotid angioplasty and stenting. J Vasc Surg. 2007;46:244–50.
Young B, Moore WS, Robertson JT, et al. An analysis of perioperative surgical mortality and morbidity in the asymptomatic carotid atherosclerosis study ACAS investigators. Asymptomatic Carotid Artheriosclerosis Study. Stroke. 1996;27:2216–24.
Leonardi M, Cenni P, Simonetti L, et al. Retrospective study of complications arising during cerebral and spinal diagnostic angiography from 1998 to 2003. Interv Neuroradiol. 2005;11:213–21.
Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke. 1990;21:209–22.
Earnest F, Forbes G, Sandok BA, et al. Complications of cerebral angiography: prospective assessment of risk. AJR Am J Roentgenol. 1984;142:247–53.
Nishanian G, Kopchok GE, Donayre CE, et al. The impact of intravascular ultrasound (IVUS) on endovascular interventions. Semin Vasc Surg. 1999;12:285–99.
Reid DB, Douglas M, Diethrich EB. The clinical value of three-dimensional intravascular ultrasound imaging. J Endovasc Surg. 1995;2:356–64.
Clark DJ, Lessio S, O’Donoghue M, et al. Safety and utility of intravascular ultrasound-guided carotid artery stenting. Catheter Cardiovasc Interv. 2004;63:355–62.
Clark DJ, Lessio S, O’Donoghue M, Tsalamandris C, Schainfeld R, Rosenfield K. Mechanisms and predictors of carotid artery stent restenosis. A serial intravascular ultrasound study. J Am Coll Cardiol. 2006;47:2390–6.
Rapp JH, Pan XM, Yu B, Swanson RA, Higashida RT, Simpson P, et al. Cerebral ischemia and infarction from atheroemboli <100 microns in size. Stroke. 2003;34:1976–80.
Hellings WE, Ackerstaff RGA, Pasterkamp G, De Vries JP, Moll FL. The carotid atherosclerotic plaque and microembolization during carotid stenting. J Cardiovasc Surg. 2006;47:115–26.
Schonholz CJ, Uflacker R, Parodi JC, Hannegan C, Selby B. Is there evidence that cerebral protection is beneficial? Clinical data. J Cardiovasc Surg (Torino). 2006;47:137–41.
Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices. A systematic review of the literature. Stroke. 2003;34:813–9.
Coppi G, Moratto R, Silingardi R, Rubino P, Sarropago G, Salemme L, et al. PRIAMUS—Proximal flow blockage cerebral protection during carotid stenting: results from a multicenter Italian registry. J Cardiovasc Surg. 2005;46:219–27.
Parodi JC, Rubin BG, Azizzadeh A, Bartoli M, Sicard GA. Endovascular treatment of an internal carotid artery thrombus using reversal of flow: a case report. J Vasc Surg. 2005;41:146–50.
Uflacker R. How to optimize carotid artery stenting. J Cardiovasc Surg (Torino). 2007;48:131–49.
Nikas D, Reith W, Schmidt A, Duda S, Mathias K, Cremonesi A, Dill H, Formgren J, Pieniazek P, Musialek P, Hornung M, Sievert H, Reimers B. Prospective, multicenter European study of the GORE flow reversal system for providing neuroprotection during carotid artery stenting. Catheter Cardiovasc Interv. 2012;80:1060–8.
Criado E, Doblas M, Fontcuberta J, Orgaz A, Flores A, Wall LP, et al. Transcervical carotid stenting with internal carotid artery flow reversal: feasibility and preliminary results. J Vasc Surg. 2004;40:476–83.
Pipinos II, Bruzoni M, Johanning JM, Longo GM, Lynch TG. Transcervical carotid stenting with flow reversal for neuroprotection: technique, results, advantages, and limitations. Vascular. 2006;14:245–55.
Matas M, Alvarez B, Ribo M, Molina C, Maeso J, Alvarez-Sabin J. Transcervical carotid stenting with flow reversal protection: experience in high-risk patients. J Vasc Surg. 2007;46(1):49–54.
Leal I, Orgaz A, Flores A, Gil J, Rodríguez R, Peinado J, Criado E, Doblas M. A diffusion-weighted magnetic resonance imaging-based study of transcervical carotid stenting with flow reversal versus transfemoral filter protection. J Vasc Surg. 2012;56:1585–90.
Cremonesi A, Rubino P, Grattoni C, Scheinert D, Castriota F, Biamino G. Multicenter experience with a new “hybrid” carotid stent. J Endovasc Ther. 2008;15:186–92.
Brown KE, Usman A, Kibbe MR, Morasch MD, Matsumura JS, Pearce WH. Carotid stenting using tapered and nontapered stents: associated neurological complications and restenosis rates. Ann Vasc Surg. 2009;23:439–45.
Geisbüsch P, Katzen BT, Peña C, Benenati JF, Uthoff H. Bivalirudin used as alternative anticoagulant in carotid artery stenting: a single center observational study. J Interv Cardiol. 2012;25:197–202.
Cogar BD, Wayangankar SA, Abu-Fadel M, Hennebry TA, Ghani MK, Kipperman RM, Chrysant GS. Clinical safety of bivalirudin in patients undergoing carotid stenting. J Invasive Cardiol. 2012;24:202–5.
Coward LJ, Featherstone RL, Brown MM. Percutaneous transluminal angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev. 2004;CD000515.
Criado E, Doblas M, Fontcuberta J, et al. Carotid angioplasty with internal carotid artery flow reversal is well tolerated in the awake patient. J Vasc Surg. 2004;40:92–7.
Chung C, Cayne NS, Adelman MA, Riles TS, Lamparello P, Han D, Marin ML, Faries PL. Improved hemodynamic outcomes with glycopyrrolate over atropine in carotid angioplasty and stenting. Perspect Vasc Surg Endovasc Ther. 2010;22:164–70.
Cayne NS, Faries PL, Trocciola SM, Saltzberg SS, Dayal RD, Clair D, Rockman CB, Jacobowitz GR, Maldonado T, Adelman MA, Lamperello P, Riles TS, Kent KC. Carotid angioplasty and stent-induced bradycardia and hypotension: Impact of prophylactic atropine administration and prior carotid endarterectomy. J Vasc Surg. 2005;41:956–61.
Mori T, Kazita K, Mima T, Mori K. Balloon angioplasty for embolic total occlusion of the middle cerebral artery and ipsilateral carotid stenting in an acute stroke stage. AJNR Am J Neuroradiol. 1999;20:1462–4.
Wholey MH, Wholey MH, Tan WA, et al. Management of neurological complications of carotid artery stenting. J Endovasc Ther. 2001;8:341–53.
Tong C, Cloft J, Joseph J, Samuels B, Dion E. Abciximab rescue in acute carotid stent thrombosis. AJNR Am J Neuroradiol. 2000;21:1750–2.
Chatuverdi S, Sohorab S, Tselis A. Carotid stent thrombosis: report of 2 fatal cases. Stroke. 2001;32:2700–2.
Ho DS, Wang Y, Chui M, et al. Intracarotid abciximab injection to abort impending ischemic stroke during carotid angioplasty. Cerebrovasc Dis. 2001;11:300–4.
Van den Berg JC, Antonius Carotid Endarterectomy, Angioplasty, and Stenting Study Group. The nature and management of complications in carotid artery stenting. Acta Chir Belg. 2004;104:60–4.
Antonius Carotid Endaterectomy Angioplasty, and Stenting Study Group. Transcranial doppler monitoring in angioplasty and stenting of the carotid bifurcation. J Endovasc Ther. 2003;10:702–10.
Macdonald S, Venables GS, Cleveland TJ, Gaines PA. Protected carotid stenting: safety and efficacy of the MedNova NeuroShield filter. J Vasc Surg. 2002;35:966–72.
Dietrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg. 1996;3:42–62.
Owen EL, Kummins NH, Bergan JJ, Sparks SR. Surgical management of acute complications and critical restenosis following carotid artery stenting. Ann Vasc Surg. 2002;16:168–75.
Vitek JJ, Roubin GS, Al-Mubarek N, New G, Iyer SS. Carotid artery stenting: technical considerations. Am J Neuroradiol. 2000;21:1736–43.
Coutts SB, Hill MD, Hu WY. Hyperperfusion syndrome: toward a stricter definition. Neurosurgery. 2003;53:1053–8.
Iwata T, Mori T, Tajiri H, Nakazaki M. Predictors of hyperperfusion syndrome before and immediately after carotid artery stenting in single-photon emission computed tomography and transcranial color-coded real-time sonography studies. Neurosurgery. 2011;68:649–55.
Disclosure
Rolando Celis and Rabih A. Chaer declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Celis, R., Chaer, R.A. Techniques for Optimizing Results in Carotid Stenting. Curr Surg Rep 1, 78–89 (2013). https://doi.org/10.1007/s40137-013-0016-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40137-013-0016-z