Abstract
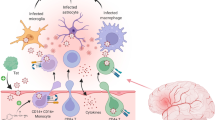
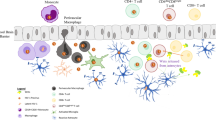
Advent of combinatorial antiretroviral therapy has widely declined the number of HIV related deaths. It has however resulted in an increase in number of people living with HIV, and their morbidity in terms of their compromised brain functions, thereby worsening the overall scenario in the form of HIV associated neurocognitive impairment that are studied under the umbrella of neuroAIDS. Productively infected macrophages “hijack” brain parenchyma, resulting in slow neurodegeneration especially in the basal ganglia, hippocampus, prefrontal cortex and white matter. Although not directly infected, neurons undergo apoptosis via different pathways as discussed in the review. In addition, more devastating is the condition when HIV synergizes with drugs of abuse and brings in oxidative stress, elevation of inflammatory cytokines and increased calcium waves ultimately leading to augmented excitotoxicity. Mother to child transmission has been another important risk factor in the field of HIV rendering the neonates afflicted with severe neurodevelopmental delays and reduced immune response. Increase in several cytokines and other molecules have been promising in early diagnosis of the syndrome, but search for a biomarker is still on. In this review, we have outlined the recent developments in the field, practical challenges in neuroAIDS research and possible future directions that may help in better management of the disease.




Similar content being viewed by others
References
Global report: UNAIDS report on the global AIDS epidemic, 2010
Department of AIDS Control, National AIDS Control Organisation, Ministry of Health & Family Welfare, Annual Report 2010–2011
Simoes EA, Babu PG, John TJ et al (1987) Evidence for HTLV-III infection in prostitutes in Tamil Nadu. Indian J Med Res 85:335–338
Antinori A, Arendt G, Becker JT et al (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799
Kaplan JE, Hanson D, Dworkin MS et al (2000) Epidemiology of Human Immunodeficiency Virus—associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 30:S5–S14
Teja VD, Talasila SR, Vemu L et al (2005) Neurologic manifestations of HIV infection: an Indian hospital-based study. AIDS Read 15(3):139–143
Deshpande AK, Patnaik MM (2005) Nonopportunistic neurologic manifestations of the human immunodeficiency virus: an Indian study. MedGenMed 7(4):2
Xu J, Ikezu T (2009) The comorbidity of HIV-associated neurocognitive disorders and Alzheimer’s disease: a foreseeable medical challenge in post-HAART era. J Neuroimmune Pharmacol 4(2):200–212
Gray F, Scaravilli F, Everall I et al (1996) Neuropathology of early HIV-1 infection. Brain Pathol 6:1–15
Valcour V, Shikuma C, Shiramizu B et al (2004) Higher frequency of dementia in older HIV-1 individuals: the Hawaii aging with HIV-1 cohort. Neurology 63(5):822–827
Bassel C, Rourke SB, Halman MH et al (2002) Working memory performance predicts subjective cognitive complaints in HIV infection. Neuropsychology 16(3):400–410
Gorman AA, Foley JM, Ettenhofer ML et al (2009) Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev 19(2):186–203
Maj M, Satz P, Janssen R et al (1994) WHO Neuropsychiatric AIDS study, cross-sectional phase II. Neuropsychological and neurological findings. Arch Gen Psychiatry 51(1):51–61
Rourke SB, Halman MH, Bassel C et al (1999) Neurocognitive complaints in HIV infection and their relationship to depressive symptoms and neuropsychological functioning. J Clin Exp Neuropsychol 21(6):737–756
Haase AT (1986) Pathogenesis of lentivirus infections. Nature 322:130–136
Peluso R, Haase A, Stowring L et al (1985) A Trojon horse mechanism for the spread of visna virus in monocytes. Virology 147:231–236
Simmons G, Reeves JD, McKnight A et al (1998) CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J Virol 72:8453–8457
Hibbitts S, Reeves JD, Simmons G et al (1999) Coreceptor ligand inhibition of fetal brain cell infection by HIV type 1. AIDS Res Hum Retroviruses 15:989–1000
Petito CK, Cash KS (1992) Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol 32:658–666
Moses AV, Nelson JA (1994) HIV infection of human brain capillary endothelial cells: implications for AIDS dementia. Adv Neuroimmunol 4:239–247
Gras G, Kaul M (2010) Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology 7:30–41
Minagar A, Shapshak P, Fujimura R et al (2002) The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci 202:13–23
Dhillon NK, Williams R, Callen S et al (2008) Roles of MCP-1 in development of HIV-dementia. Front Biosci 13:3913–3918
Toborek M, Lee YW, Flora G et al (2005) Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol 25:181–199
Sporer B, Koedel U, Paul R et al (2000) Human immunodeficiency virus type-1 Nef protein induces blood–brain barrier disruption in the rat: role of matrix metalloproteinase-9. J Neuroimmunol 102:125–130
Williams KC, Corey S, Westmoreland SV et al (2001) Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med 193:905–915
Cosenza MA, Zhao ML, Si Q et al (2002) Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol 12:442–455
Gorry PR, Ong C, Thorpe J et al (2003) Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res 1:463–473
Shi B, Girolami UD, He J et al (1996) Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Investig 98:1979–1990
Xu Y, Kulkosky J, Acheampong E et al (2004) HIV-1-mediated apoptosis of neuronal cells: proximal molecular mechanisms of HIV-1-induced encephalopathy. PNAS 101(18):7070–7075
Levi G, Patrizio M, Bernardo A et al (1993) Human immunodeficiency virus coat protein gp120 inhibits the beta-adrenergic regulation of astroglial and microglial functions. PNAS 90(4):1541–1545
Benos DJ, Hahn BH, Shaw GM et al (1994) gp120-mediated alterations in astrocyte ion transport. Adv Neuroimmunol 4(3):175–179
Conant K, Demo AG, Nath A et al (1998) Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. PNAS 95(6):3117–3121
Cinque P, Vago L, Mengozzi M et al (1998) Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. Aids 12(11):1327–1332
Rumbaugh J, Turchan-Cholewo J, Galey D et al (2006) Interaction of HIV Tat and matrix metalloproteinase in HIV neuropathogenesis: a new host defense mechanism. FASEB J 20(10):1736–1738
Haughey NJ, Mattson MP (2002) Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr 31(Suppl 2):S55–S61
Eugenin EA, King JE, Nath A et al (2007) HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. PNAS 104(9):3438–3443
Qi M, Aiken C (2008) Nef enhance HIV-1 infectivity via association with the virus assembly complex. Virology 373(2):287–297
Levy DN, Refaeli Y, Weiner DB (1995) Extracellular Vpr protein increases cellular permissiveness to human immunodeficiency virus replication and reactivates virus from latency. J Virol 69(2):1243–1252
Patel CA, Mukhtar M, Pomerantz RJ (2000) Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J Virol 74:9717–9726
Lannuzel A, Lledo PM, Lamghitnia HO et al (1995) HIV-1 envelope proteins gp120 and gp160 potentiate NMDA-induced [Ca2+]i increase, alter [Ca2+]i homeostasis and induce neurotoxicity in human embryonic neurons. Eur J Neurosci 7(11):2285–2293
Toggas SM, Masliah E, Mucke L et al (1996) Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res 706(2):303–307
Epstein LG, Gelbard HA (1999) HIV-1-induced neuronal injury in the developing brain. J Leukoc Biol 65(4):453–457
Okamoto S, Kang YJ, Brechtel CW et al (2007) HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell 1(2):230–236
Krathwohl MD, Kaiser JL (2004) HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis 190(2):216–226
Mishra M, Taneja M, Malik S et al (2010) Human immunodeficiency virus type 1 Tat modulates proliferation and differentiation of human neural precursor cells: implication in neuroAIDS. J Neurovirol 16(5):355–367
Geretti AM (2006) HIV-1 subtypes: epidemiology and significance for HIV management. Curr Opin Infect Dis 19:1–7
Gurtlerr LG, Zekeng L, Tsague JM et al (1996) HIV-1 subtype O: epidemiology, pathogenesis, diagnosis and perspectives of the evolution of HIV. Arch Virol Suppl 11:195–202
Simon F, Mauclere P, Roques P et al (1998) Identification of a new human immune-deficiency virus type 1 distinct from group M and group O. Nat Med 4(9):1032–1037
Hu D, DonderoTJ, Mastro TD et al (1998) In: Wormser GP (ed) Global and molecular epidemiology of HIV. pp 27–40
Wainberg MA (2004) HIV-1 subtype distribution and the problem of drug resistance. AIDS 18(Suppl 3):S63–S68
Siddappa NB, Dash PK, Mahadevan A et al (2004) Identification of subtype C human immunodeficiency virus type 1 by subtype-specific PCR and its use in the characterization of viruses circulating in the southern parts of India. J Clin Microbiol 42(6):2742–2751
Tripathi SP, Kulkarni SS, Jadhav SD et al (2005) Subtype B and subtype C HIV type 1 recombinants in the northeastern state of Manipur, India. AIDS Res Hum Retroviruses 21(2):152–157
Gupta JD, Satishchandra P, Gopukumar K et al (2007) Neuropsychological deficits in human immunodeficiency virus type 1 clade C-seropositive adults from South India. J Neurovirol 13(3):195–202
Riedel D, Ghate M, Nene M et al (2006) Screening for HIV dementia in an HIV-infected population in India. J Neurovirol 12(1):34–38
Ranga U, Shankarappa R, Siddappa NB et al (2004) Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virol 78(5):2586–2590
Li W, Huang Y, Reid R et al (2008) NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci 28(47):12190–12198
Campbell GR, Watkins JD, Singh KK et al (2007) Human immunodeficiency virus type 1 subtype C Tat fails to induce intracellular calcium flux and induces reduced tumor necrosis factor production from monocytes. J Virol 81(11):5919–5928
Mishra M, Vetrivel S, Siddappa NB et al (2008) Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: significance of dicysteine C30C31 motif. Ann Neurol 63(3):366–376
Rao VR, Sas AR, Eugenin EA et al (2008) HIV-1 clade-specific differences in the induction of neuropathogenesis. J Neurosci 28(40):10010–10016
Campbell GR, Loret EP, Spector SA et al (2010) HIV-1 clade B Tat, but not clade C Tat, increases X4 HIV-1 entry into resting but not activated CD4+ T cells. J Biol Chem 285(3):1681–1691
Wong JK, Campbell GR, Spector SA et al (2010) Differential induction of interleukin-10 in monocytes by HIV-1 clade B and clade C Tat proteins. J Biol Chem 285(24):18319–18325
Gandhi N, Saiyed Z, Thangavel S et al (2009) Differential effects of HIV type 1 clade B and clade C Tat protein on expression of proinflammatory and antiinflammatory cytokines by primary monocytes. AIDS Res Hum Retroviruses 25(7):691–699
Samikkannu T, Rao KV, Gandhi N et al (2010) Human immunodeficiency virus type 1 clade B and C Tat differentially induce indoleamine 2,3-dioxygenase and serotonin in immature dendritic cells: implications for neuroAIDS. J Neurovirol 16(4):255–263
Nath A, Hauser KF, Wojna V et al (2002) Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr 31(Suppl 2):S62–S69
Solomon SS, Hawcroft CS, Narasimhan P et al (2008) Comorbidities among HIV-infected injection drug users in Chennai, India. Indian J Med Res 127:447–452
Hauser KF, Hahn YK, Adjan VV et al (2009) HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia 57(2):194–206
Hauser KF, El-Hage N, Buch S et al (2005) Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res 8(1–2):63–80
El-Hage N, Gurwell JA, Singh IN et al (2005) Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia 50(2):91–106
El-Hage N, Wu G, Wang J et al (2006) HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia 53(2):132–146
Mahajan SD, Aalinkeel R, Reynolds JL et al (2005) Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr HIV Res 3(3):277–288
El-Hage N, Bruce-Keller AJ, Yakovleva T et al (2008) Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription. PLoS One 3(12):e4093
Peteron PK, Sharp BM, Gekker G et al (1990) Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS 4(9):869–873
Bagasra O, Pomerantz RJ (1993) Human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells in the presence of cocaine. J Infect Dis 168(5):1157–1164
Roth MD, Tashkin DP, Choi R et al (2002) Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J Infect Dis 185(5):701–705
Fitting S, Xu R, Bull C et al (2010) Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol 177(3):1397–1410
Khurdayan VK, Buch S, El-Hage N et al (2004) Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci 19(12):3171–3182
Turchan JC, Dimayuga FO, Gupta S et al (2009) Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. J Neurochem 108(1):202–215
Santos A, Cremades R, Rodriguez JC et al (2008) Mycobacterium peregrinum: bactericidal activity of antibiotics alone and in combination. J Infect Chemother 14(3):262–263
Pitcher J, Shimizu S, Burbassi S et al (2010) Disruption of neuronal CXCR4 function by opioids: preliminary evidence of ferritin heavy chain as a potential etiological agent in neuroAIDS. J Neuroimmunol 224(1–2):66–71
Avdoshina V, Biggio F, Palchik G et al (2010) Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia 58(13):1630–1639
Li Y, Wang X, Tian S et al (2002) Methadone enhances human immunodeficiency virus infection of human immune cells. J Infect Dis 185(1):118–122
Potula R, Persidsky Y (2008) Adding fuel to the fire: methamphetamine enhances HIV infection. Am J Pathol 172(6):1467–1470
Nair MP, Mahajan SD, Schwartz SA et al (2005) Cocaine modulates dendritic cell-specific C type intercellular adhesion molecule-3-grabbing nonintegrin expression by dendritic cells in HIV-1 patients. J Immunol 174(11):6617–6626
Chana G, Everall IP, Crews L et al (2006) Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology 67(8):1486–1489
Wilson JM, Kalasinsky KS, Levey AI et al (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2(6):699–703
Wilson JM, Levey AI, Bergeron C et al (1996) Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol 40(3):428–439
Villemagne V, Yuan J, Wong DF et al (1998) Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C] WIN-35, 428 positron emission tomography studies and direct in vitro determinations. J Neurosci 18(1):419–427
Turchan J, Anderson C, Hauser KF et al (2001) Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci 2:3
Brown JM, Yamamoto BK (2003) Effects of amphetamines on mitochondrial function: role of free radicals and oxidative stress. Pharmacol Ther 99:45–53
Keller MA, Venkatraman TN, Thomas A et al (2004) Altered neurometabolite development in HIV-infected children: correlation with neuropsychological tests. Neurology 62(10):1810–1817
Boivin MJ, Green SD, Davies AG et al (1995) A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol 14(1):13–21
Dollard SC, James HJ, Sharer LR et al (1995) Activation of nuclear factor kappa B in brains from children with HIV-1 encephalitis. Neuropathol Appl Neurobiol 21(6):518–528
NACO news Oct–Dec 2006. Volume 2, issue 4
Spector SA (2001) Mother-to-infant transmission of HIV-1: the placenta fights back. J Clin Invest 107(3):267–269
Van AR, Harrington PR, Dow A et al (2007) Neurologic and neuro developmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol 11(1):1–9
Kamat A, Ravi V, Desai A et al (2009) Estimation of virological and immunological parameters in subjects from South India infected with human immunodeficiency virus type 1 clade C and correlation of findings with occurrence of neurological disease. J Neurovirol 15(1):25–35
Huang JS, Letendre S, Marquie-Beck J et al (2007) Low CSF leptin levels are associated with worse learning and memory performance in HIV-infected men. J Neuroimmune Pharmacol 2:352–358
Haughey NJ, Cutler RG, Tamara A et al (2004) Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol 55(2):257–267
Roberts TK, Eugenin EA, Morgello S et al (2010) PrPC, the Cellular isoform of the human prion protein, is a novel biomarker of HIV-associated neurocognitive impairment and mediates neuroinflammation. Am J Pathol 176(6):2819–2830
Rezk NL, Tidwell RR, Kashuba AD (2003) Simultaneous determination of six HIV nucleoside analogue reverse transcriptase inhibitors and nevirapine by liquid chromatography with ultraviolet absorbance detection. J Chromatogr 791:137–147
De-Clercq E (2004) Non-nucleoside reverse transcriptase inhibitors (NNRTIs): past, present, and future. Chem Biodivers 1:44–64
Lalezari JP, Henry K, O’Hearn M et al (2003) Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med 348:2175–2185
Yost R, Pasquale TR, Sahloff EG (2009) Maraviroc: a coreceptor CCR5 antagonist for management of HIV infection. Am J Health Syst Pharm 66:715–726
Barry M, Gibbons S, Back D et al (1997) Protease inhibitors in patients with HIV disease. Clinically important pharmacokinetic considerations. Clin Pharmacokinet 32:194–209
Serrao E, Odde S, Ramkumar K et al (2009) Raltegravir, elvitegravir, and metoogravir: the birth of “me-too” HIV-1 integrase inhibitors. Retrovirology 6:25
Department of AIDS Control Ministry of Health and Family Welfare Government of India. Annual Report 2008–2009
Hogg R, Lima V, Sterne JA et al (2008) Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 372:293–299
Harrison KM, Song R, Zhang X (2010) Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr 53:124–130
Liner KJ, Hall CD, Robertson KR et al (2008) Effects of antiretroviral therapy on cognitive impairment. Curr HIV/AIDS Rep 5(2):64–71
Sinha S, Mathews T, Arunodaya GR et al (2004) HIV-1 clade-C-associated “ALS”-like disorder: first report from India. J Neurol Sci 224(1–2):97–100
McArthur JC, Brew BJ, Nath A et al (2005) Neurological complications of HIV infection. Lancet Neurol 4(9):543–555
Heseltine PN, Goodkin K, Atkinson JH et al (1998) Randomized double-blind placebo-controlled trial of peptide T for HIV-associated cognitive impairment. Arch Neurol 55(1):41–51
Lipton SA, Chen HS (2004) Paradigm shift in neuroprotective drug development: clinically tolerated NMDA receptor inhibition by memantine. Cell Death Differ 11(1):18–20
Evans SR, Yeh TM, Sacktor N et al (2007) Selegiline transdermal system (STS) for HIV-associated cognitive impairment: open-label report of ACTG 5090. HIV Clin Trials 8(6):437–446
Eggert D, Dash PK, Gorantla S et al (2010) Neuroprotective activities of CEP-1347 in models of neuroAIDS. J Immunol 184(2):746–756
Horberg MA, Silverberg MJ, Hurley LB et al (2008) Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr 47:384–390
Everall IP, Bell C, Mallory M et al (2002) Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci 21:493–501
Smith SM (2005) Valproic acid and HIV-1 latency: beyond the sound bite. Retrovirology 2:56
Letendre S, Woods S, Ellis R et al (2006) Lithium improves HIV-associated neurocognitive impairment. AIDS 20:1885–1888
Kaul M, Lipton SA (2005) Experimental and potential future therapeutic approaches for HIV-1 associated dementia targeting receptors for chemokines, glutamate and erythropoietin. Neurotox Res 8(1–2):167–186
Dou H, Grotepas CB, McMillan JM et al (2009) Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol 183(1):661–669
Saiyed ZM, Gandhi NH, Nair MP et al (2010) Magnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood-brain barrier. Int J Nanomed 5:157–166
Hütter G, Nowak D, Mossner M et al (2009) Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 360(7):692–698
Acknowledgments
The research work of Dr. Pankaj Seth at NBRC is supported by research grants from Department of Biotechnology (DBT), New Delhi, India, National Institutes of Health (NIH RO1), Bethesda, USA and institutional core funding. Research Fellowship to MP and PG by Council of Scientific and Industrial Research, New Delhi, is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manju Pant and Pretty Garg contributed equally.
Rights and permissions
About this article
Cite this article
Pant, M., Garg, P. & Seth, P. Central Nervous System Infection by HIV-1: Special Emphasis to NeuroAIDS in India. Proc. Natl. Acad. Sci. Sect B. Biol. Sci. 82, 81–94 (2012). https://doi.org/10.1007/s40011-011-0007-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-011-0007-8