Abstract
Purpose
This study was designed to compare the efficacy of polymyxin B with other antimicrobials in the treatment of ventilator-associated pneumonia (VAP) and tracheobronchitis (VAT) by Pseudomonas aeruginosa or Acinetobacter baumannii.
Methods
A prospective cohort study was performed. Patients >18 years of age with the diagnosis of VAP or VAT who received appropriate therapy for >48 h were analyzed. The primary outcome was 30-day mortality. Clinical covariates were assessed and compared between the groups.
Results
A total of 67 episodes were analyzed: 45 (67 %) treated with polymyxin B and 22 (33 %) with comparators. The crude 30-day mortality was 53 % (24 of 45) in the polymyxin B group and 27 % (6 of 22) in the comparator group (P = 0.08). Multivariable analysis using Cox regression models indicated that polymyxin B treatment was independently associated with increased mortality.
Conclusions
Polymyxin B treatment in the currently recommended dosage may be inferior to other drugs in the treatment of VAP and VAT caused by organisms tested as susceptible in vitro to this agent.
Similar content being viewed by others

Introduction
Ventilator-associated pneumonia (VAP) and ventilator-associated tracheobronchitis (VAT) are among the most common infections in critically ill patients [1]. VAP is especially associated with elevated mortality and increased length of hospital stay and costs [2]. Pseudomonas aeruginosa and Acinetobacter baumannii are major causes of both VAP and VAT [3]. Treatment of infections by such bacteria are usually a challenge and many isolates are only susceptible to polymyxins, polymyxin B, and colistin, which are antibiotics that re-emerged in later years as the last-resort therapy for the treatment of multidrug-resistant (MDR) Gram-negative bacteria [3–5].
The use of polymyxins has been supported by the lack of other treatment options and by case series suggesting that these drugs are efficacious and safe [5, 6]. Recently, however, three large studies have shown that treatment with polymyxins, both colistin and polymyxin B, were inferior to comparators for the treatment of serious nosocomial infections [7–9].
Polymyxins are most commonly prescribed for the treatment of MDR Gram-negative bacteria causing respiratory infections, particularly VAP and VAT. The aim of this study was to compare the efficacy of polymyxin B with other antibiotics in the treatment of these latter conditions caused by P. aeruginosa or A. baumannii.
Methods
Study design
A single-center prospective cohort study was performed, from February 2009 to December 2010, at a 600-bed teaching hospital, Hospital São Lucas (HSL), in Porto Alegre, Brazil. All patients admitted to the 13-bed intensive care unit (ICU) of HSL, submitted to mechanical ventilation for at least 48 h, who had growth of P. aeruginosa or A. baumannii (≥105 cfu/mL) from quantitative tracheal aspirates (QTA), and clinical diagnosis of VAP or VAT were enrolled for the study. They were excluded if they were <18 years old, if they had received treatment for <48 h or died in this period, or if they have not received appropriate therapy. Patients were assigned to the polymyxin B or other antimicrobial group according to their first appropriate treatment, if this treatment was administered for at least 48 h. QTA were collected, daily, during the first 15 days of treatment for the evaluation of microbiological outcomes. The decision to treat and the dosage regimes were at the discretion of the attendant physician. The study was approved by the local ethics committee. A consent term was signed by a family member or other legal representative.
Variables and definitions
The primary outcome was 30-day mortality, defined as death for any cause during the first 30 days after the onset of infection. The onset of infection was defined as the day that the QTA that resulted in the growth of P. aeruginosa or A. baumannii was collected. Secondary outcomes were length of mechanical ventilation after the initiation of appropriate treatment; incidence of superinfection, defined by the growth of another microorganism during the first 15 days after treatment; and eradication of the bacteria from respiratory secretions, defined as the absence of growth in at least one QTA and no growth in subsequent examinations. VAP was defined as the presence of a radiographic infiltrate that was new or progressive, along with the presence of two or more of the following criteria: fever (temperature >38 °C) or hypothermia (temperature <36 °C), purulent sputum, leukocytosis (>10,000 cells/mm3), or leukopenia (<4,000 cells/mm3) [2]; VAT was defined as the presence of purulent secretions and one of the following: fever or hypothermia, and leukocytosis or leukopenia, as defined above [1]. Respiratory secretion was considered to be purulent if >25 neutrophils and <10 epithelial cells per high-power field were present. Appropriate therapy was defined as the administration of an antimicrobial agent with in vitro susceptibility.
Covariables potentially associated with mortality were evaluated. Adequate dosage regimes of the drugs was defined as a total daily dose of at least 6 g of cefepime and ceftazidime (divided into three doses), 1,200 mg of ciprofloxacin (divided into two or three doses), 750 mg of levofloxacin (single daily dose), 2 g of imipenem (divided into four doses), 3 g of meropenem (divided into three doses), and 13.5 g of piperacillin–tazobactam (divided into three or four doses), or adjusted as indicated in the product drug information package insert. Adequate dosage regimes of polymyxin B was not defined and median doses were analyzed separately. The institutional protocol for this drug recommends the administration of 2.5 mg/kg/day every 12 h (1 mg = 10,000 U). Dose adjustment of polymyxin B for renal impairment was not considered since total body clearance of this drug is not affected by creatinine clearance according to a recent pharmacokinetics study [10].
Microbiology
All isolates were identified by the Vitek system (bioMérieux, Marcy l’Etoile, France). Susceptibility was determined by the disk diffusion method and the results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) [11]. All first and subsequent isolates recovered from respiratory secretions were initially planned to have minimum inhibitory concentrations (MICs) for antimicrobials evaluated by broth microdilution to assess the development of resistance during treatment. However, owing to storage problems, only 19 (seven P. aeruginosa and 12 A. baumannii) isolates of 67 episodes of VAP or VAT obtained during the study could be recovered for further analysis. Thus, MICs were determined only in these isolates. For this reason, all non-tested A. baumannii isolates were considered to be susceptible to polymyxin B. Isolates with MIC ≤2 mg/L were susceptible [11].
Statistical analysis
All statistical analyses were carried out using SPSS for Windows, version 16.0. Variables potentially associated with the outcome were compared between polymyxin B and comparator groups (all other antimicrobial drugs), as well as among 30-day survivors and non-survivors. P-values were calculated using the Chi-square or Fisher’s exact tests for categorical variables and the Student’s t or Wilcoxon–Mann–Whitney tests for continuous variables. In the bivariate analysis, patients discharged before 30 days were considered to be alive; in the multivariate analysis, they were censored in such date. Variables for which the P-value was ≤0.20 in the bivariate analysis were included one by one using a forward stepwise method in a Cox regression model according their P value, with time to the event (death) as the outcome. Variables were checked for confounding and collinearity. A P ≤ 0.10 was set as the limit for acceptance and a P > 0.10 for the removal of new terms in the model. Finally, variables with a P ≤ 0.20 in the bivariate analysis of polymyxin B and beta-lactam groups were forced into the model to adjust for any potential residual confounding. Proportional hazards assumption was graphically checked inspecting the log[−log(S)] plot. All tests were two-tailed and a P-value ≤ 0.05 was considered to be significant.
Subgroup analysis of patients with VAP was a priori defined. The variables included in the final model of this subgroup were the same as in the final model.
Results
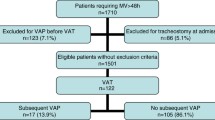
Seventy-seven patients met the criteria for VAP (57) or VAT (20) and had the growth of ≥105 cfu/mL P. aeruginosa or A. baumannii isolates in QTA. Of these, 14 (18.0 %) were excluded: 3 (21.4 %) did not receive appropriate treatment and 11 (78.6 %) died within <48 h after the onset of appropriate therapy, resulting in a total of 63 patients. Of these 63 patients, three had more than one episode of VAP or VAT during the period of the study, resulting in 67 episodes analyzed. The time between the first and second episodes in these four patients was 38, 42, 56, and 360 days, respectively.
There were 54 (80.6 %) episodes of VAP (23 by P. aeruginosa, 28 by A. baumannii, and three by both) and 13 (19.4 %) episodes of VAT (five by P. aeruginosa, seven by A. baumannii, and one by both). In 45 (67.2 %) episodes, patients were treated with polymyxin B and in 22 (32.8 %), they were treated with comparators: 4 (18.2 %) with ceftazidime, 4 (18.2 %) with meropenem, 4 (18.2 %) with cefepime, 3 (13.6 %) with piperacillin–tazobactam, 3 (13.6 %) with ciprofloxacin, 3 (13.6 %) with levofloxacin, and 1 (4.5 %) with imipenem. The median (interquartile range) total daily dose of polymyxin B was 150 mg (150–200 mg; 1 mg = 10,000 U), administered every 12 h. There was no statistically significant difference in the total daily doses of polymyxin B among 30-day survivors and non-survivors (P = 0.65). Twenty (90.9 %) patients in the comparator group received adequate dosage regimens. Two (4.4 %) patients in the polymyxin B group changed their treatment for a beta-lactam after 4–7 days after starting therapy and 8 (36.4 %) changed their treatment to polymyxin B after 4–11 days after starting therapy. Polymyxin B and other antimicrobial groups were comparable in most of the variables assessed, but there was a tendency for higher frequency of males, other infections, and higher mean APACHE II score at ICU admission in the polymyxin B group (Table 1).
All P. aeruginosa isolates were susceptible to polymyxin B by the disk diffusion method, and the seven isolates recovered for analysis have their MIC for polymyxin B ranging from 0.5 to 2.0 mg/L. Twelve A. baumannii isolates had their MICs for polymyxin B ranging from 0.5 to 1 mg/L.
The overall 30-day mortality was 44.8 % (30 of 67): 53.3 % (24 of 45) in the polymyxin B group and 27.3 % (6 of 22) in the comparator group [relative risk (RR) 1.96; 95 % confidence interval (CI) 0.94–4.08, P = 0.08]. The mortality rates in the polymyxin B group was 25.7 per 1,000 patient-days and 10.3 per 1,000 patient-days in the comparator group (P = 0.03). All but three of 37 patients who were classified as 30-day survivors were followed-up to day 30. Two of the three patients were followed-up for 4–24 days (polymyxin B group) and one for 10 days (comparator group). The bivariate analysis of the factors potentially associated with 30-day mortality is shown in Table 2.
The results of the final multivariate model demonstrated that the use of polymyxin B, length of hospital stay before infection, and development of renal impairment during therapy (≥100 % increase of creatinine from baseline levels) were independently associated with higher 30-day mortality (Table 3). APACHE II score at the onset of infection were maintained in the final model, regardless of the absence of statistical significance for the adjustment of potential residual confounding.
Twenty-four (44.4 %) of the 54 patients in the subgroup with VAP died within 30 days: 19 (54.3 %) in the polymyxin B-treated group and 5 (26.3 %) in the comparator group (RR 2.06; 95 % CI 0.92–4.64, P = 0.09). After adjustment, polymyxin B was independently associated with 30-day mortality as the length of hospital stay before infection and the development of renal impairment during therapy (Table 3).
A per-protocol analysis was performed, excluding the ten patients who changed their treatment during the first 15 days. The overall 30-day mortality was 47.4 % (27 of 57): 55.8 % (24 of 43) in the polymyxin B group and 21.4 % (3 of 14) in the comparator group (RR 2.60; 95 % CI 0.92–7.35, P = 0.054). The results of the multivariate model with the same variables as the final model are displayed in Table 3.
There were no statistically significant differences in secondary outcomes between both groups (Table 4). Eradication rates of A. baumannii isolates from respiratory secretions were significantly higher (65.7 %; 23 of 35) compared with P. aeruginosa (11.1 %; 3 of 27), P < 0.001. Patients with infection by both organisms eradicated only A. baumannii, but not P. aeruginosa. Patients who presented bacterial eradication tended to have lower 30-day mortality rates than those who did not eradicate (9 of 26, 34.6 % vs. 21 of 68, 55.3 %, respectively).
Discussion
Our study showed that patients with VAP or VAT caused by P. aeruginosa or A. baumannii treated with polymyxin B had higher 30-day mortality than those treated with other antimicrobials, mostly beta-lactams. Our results point toward the same direction of other recent studies which suggest that polymyxins might be inferior to other drugs in the treatment of severe infections by Gram-negative bacilli [7–9]. Additionally, other recent studies with KPC-producing organisms have also demonstrated that therapy with polymyxins alone is inferior to polymyxins in combination with other antimicrobials in the treatment of KPC-producing Enterobacteriaceae [15, 16]. It was not our objective and only a few patients received combination regimes in polymyxin B group; thus, we could not demonstrate any superiority of such an approach in our study.
Among covariables, only ≥100 % increase of creatinine from baseline levels during therapy and length of hospital stay before the infection were significantly associated with 30-day mortality. The occurrence of ≥100 % increase of creatinine was not distinct between groups and the effect of polymyxin B therapy was adjusted for this variable in the multivariate model; thus, this toxicity was not the cause of poorer outcomes seen in the polymyxin B group. We believe that the length of hospital stay before infection is likely reflecting, to some degree, the severity of the illness that might not be fully captured by APACHE II and SOFA scores. It is important to highlight that higher mortality rates for polymyxin B remained after such adjustments. APACHE II score at the onset of infection was maintained in the final model, regardless of the lack of statistical significance, to control for a potential residual confounding caused by severity of illness. The risk was very similar in the model not containing this latter variable (data not shown).
A potential factor corroborating to adverse outcomes in the polymyxin B group may be the appropriateness of dosage regimes. Although we have previously found that ≥200 mg/day of polymyxin B was associated with lower in-hospital mortality [17], the adequate dosage regime of this drug is not fully defined. Thus, we did not perform a categorization of adequate and non-adequate dosage for this drug. However, although dosage was not significantly associated with 30-day mortality in bivariate analysis in the polymyxin B group, considering the 200 mg cut-off, only 46.7 % of the 45 patients in this group received ≥200 mg/day (data not shown), and it might be possible that it has affected the outcomes.
In our study, patients were allocated into polymyxin B or comparator groups according to their first appropriate treatment. Actually, this design tended to favor the null hypothesis. Only 2 (4.4 %) patients in the polymyxin B group changed their therapy to beta-lactams, but 8 (36.4 %) changed their treatment to polymyxin B. However, as stated above, these changes would favor the null hypothesis, i.e., the absence of a difference between groups, since some patients in the comparator arm have received the “inferior” treatment and others in the polymyxin B arm have received the “superior” treatment. Indeed, the per-protocol analysis showed very similar results.
The main limitation of our study was that we could not assess polymyxin B susceptibility in all A. baumannii isolates. This has occurred because most isolates were lost owing to storage problems. Indeed, polymyxin B resistance could explain poorer outcomes in patients from this group. Fortunately, recent studies have shown that polymyxin B resistance among A. baumannii is still very low (<1 %) [4], and this is the exact rate seen in other current studies which have been performed in hospitals from our city (unpublished data). Additionally, none of the tested A. baumannii isolates in our study presented an MIC higher than 1 mg/L. Another limitation is that the study was conducted in a setting of high incidence of MDR P. aeruginosa and A. baumannii, and only 22 patients could be assessed in the comparator group. This might have decreased the power to detect differences in some covariates between groups, but we believe that, if it has had some influence in the results, it would not fully explain our main findings. Anyway, as stated above, we tried to decrease the influence of any residual confounding by severity of illness by forcing the APACHE II score into the final model, regardless of statistical significance.
Finally, there were no differences in secondary outcomes, including bacterial eradication. Interestingly, bacterial eradication did not occur in many patients with favorable clinical outcomes, especially in patients infected by P. aeruginosa, a fact that has already been observed in previous studies [18–20]. Although not statistically significant, a trend to lower mortality rates was observed in patients who eradicated the bacteria from respiratory secretion, a fact that might be related to treatment efficacy.
In summary, this is the first study suggesting that polymyxin B therapy may be inferior to other antimicrobials in the treatment of VAP or VAT caused by P. aeruginosa and A. baumannii. Our results should not be taken as definitive but they point towards the same direction of other recent comparative studies, which found poorer outcomes in patients treated with polymyxins when compared to other antimicrobials. This study further highlights the need for improving the knowledge on the pharmacokinetics/pharmacodynamics of this rescue drug in order to optimize its clinical use and the need for additional studies assessing the potential superiority of combinations of other antimicrobials with polymyxins to improve outcomes in patients with VAT and VAP.
References
Craven DE, Hjalmarson KI. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin Infect Dis. 2010;51(Suppl 1):S59–66.
American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
Zavascki AP, Carvalhaes CG, Picão RC, Gales AC. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71–93.
Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY antimicrobial surveillance program (2006–09). J Antimicrob Chemother. 2011;66:2070–4.
Zavascki AP, Li J. Intravenous colistimethate for multidrug-resistant Gram-negative bacteria. Lancet Infect Dis. 2008;8:403–5.
Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007;60:1206–15.
Kvitko CH, Rigatto MH, Moro AL, Zavascki AP. Polymyxin B versus other antimicrobials for the treatment of Pseudomonas aeruginosa bacteraemia. J Antimicrob Chemother. 2011;66:175–9.
Oliveira MS, Prado GV, Costa SF, Grinbaum RS, Levin AS. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. J Antimicrob Chemother. 2008;61:1369–75.
Paul M, Bishara J, Levcovich A, Chowers M, Goldberg E, Singer P, et al. Effectiveness and safety of colistin: prospective comparative cohort study. J Antimicrob Chemother. 2010;65:1019–27.
Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, et al. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis. 2008;47:1298–304.
Clinical Laboratory Standards Institute (CLSI) (2009) Performance standards for antimicrobial susceptibility testing; 19th informational supplement (M100-S19). Wayne, PA.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011;17:1798–803.
Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–13.
Elias LS, Konzen D, Krebs JM, Zavascki AP. The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J Antimicrob Chemother. 2010;65:2231–7.
El Solh AA, Akinnusi ME, Wiener-Kronish JP, Lynch SV, Pineda LA, Szarpa K. Persistent infection with Pseudomonas aeruginosa in ventilator-associated pneumonia. Am J Respir Crit Care Med. 2008;178:513–9.
Kiem S, Schentag JJ. Impact of organism species on microbial eradication and development of resistance in severe gram-negative pneumonia. J Chemother. 2010;22:103–9.
Zavascki AP, Li J, Nation RL, Superti SV, Barth AL, Lutz L, et al. Stable polymyxin B susceptibility to Pseudomonas aeruginosa and Acinetobacter spp. despite persistent recovery of these organisms from respiratory secretions of patients with ventilator-associated pneumonia treated with this drug. J Clin Microbiol. 2009;47:3064–5.
Acknowledgments
This study was supported by grants from Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (08-494).
Conflict of interest
A.P.Z. is a research fellow (301829/2008-0) and D.K. has received scientific initiation fellowship (507318/2010-2) from the National Council for Scientific and Technological Development (CNPq), Ministry of Science and Technology, Brazil. All other authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rigatto, M.H., Ribeiro, V.B., Konzen, D. et al. Comparison of polymyxin B with other antimicrobials in the treatment of ventilator-associated pneumonia and tracheobronchitis caused by Pseudomonas aeruginosa or Acinetobacter baumannii . Infection 41, 321–328 (2013). https://doi.org/10.1007/s15010-012-0349-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-012-0349-z