Abstract
Background
Malnutrition is a frequent complication in patients with cancer and can negatively affect the outcome of treatments. On the other hand, side effects of anticancer therapies can also lead to inadequate nutrient intake and subsequent malnutrition. The nutritional screening aims to identify patients at risk of malnutrition for prompt treatment and/or careful follow-up.
Methods and results
This manuscript highlights the need of an interdisciplinary approach (oncologist, nutritionist, dietitian, psychologist, etc.) to empower patients who are experiencing loss of physiological and biological function, fatigue, malnutrition, psychological distress, etc., as a result of cancer disease or its treatment, and maintain an acceptable quality of life.
Conclusions
It is necessary to make all healthcare professionals aware of the opportunity to identify cancer patients at risk of malnutrition early in order to plan the best possible intervention and follow-up during cancer treatment and progression.
Similar content being viewed by others
1 Introduction
Cancer is a systemic disease that directly affects the region of onset and can metastasize to other sites, causing a variety of complications and loss of progressive organ function. The development of the disease may be initially slow or rapidly evolving, unavoidably affecting nutritional status [1].
Malnutrition is a possible complication in patients with cancer and can be the first symptom to reveal the presence of the disease. Even before starting anticancer treatment, patients can experience profound metabolic and physiological alterations with increased needs of macro- and micronutrients [2]. Data on the prevalence of malnutrition vary broadly depending on the evaluation criteria such as tumor type, site, and extension, as well as anticancer treatment. The prevalence of malnutrition among cancer patients has been estimated to range between 15% and 80% [3]; the main symptoms embrace weight loss and asthenia of different degrees. Malnutrition can negatively affect the clinical decision to resect the tumor, which is the main and potentially curative step in the management of cancer. Indeed, malnutrition can increase the incidence of postoperative complications, such as delayed wound healing, dehiscence of anastomosis, morbidity, and mortality [4].
Medical treatment of cancer patients usually focuses on the administration of cytotoxic agents and/or radiation therapy. These tools can potentially eradicate or reduce tumor size, but may have several toxic side effects that in turn can also weaken the patient, particularly by decreasing appetite or inducing nausea and vomiting, fatigue, and asthenia. When malnutrition establishes, it can be necessary to reduce the dose of cytotoxic agents and/or modify the radiation timing between temporary or definite cessation of treatment. Direct associations have been reported between the necessity to stop or delay anticancer treatment and a reduction in the time of remission, in overall survival and in global response rates to radio/chemotherapy [5]. In addition, malnutrition is an independent risk factor for quality of life. Malnutrition impairs the immune status and reduces the body’s defense against infectious diseases [6]. In light of these possible complications, malnutrition represents a poor prognostic factor and, as such, should be prevented or detected as early as possible [7].
There are limited data in the literature on the effectiveness of nutritional screening and early treatment of malnutrition [8, 9]. An interdisciplinary approach (oncologist, nutritionist, nurse, dietitian, physical therapist, psychologist, etc.) is necessary for patients who are experiencing loss of physiological or biological function, fatigue, malnutrition, psychological distress, and other symptoms as a result of cancer disease or its treatment. The aim of such an approach is to improve their quality of life. Our manuscript aims to enhance attention of professional healthcare providers and to encourage further studies into this topic.
2 Etiology of malnutrition
Weight loss in cancer patients is due to diverse factors, among them the production of inflammatory and catabolic mediators playing important roles. Such markers include acute-phase proteins, interleukin-6 (IL-6), or the ubiquitine–proteasome complex whose activity may be increased by the neoplasm itself, particularly in some rapidly growing tumors such as those of the pancreas or the lungs [10]. The neoplastic mass can represent a possible mechanical obstruction to the way in the digestive tract, causing dysphagia and impaired swallowing (head-neck, esophageal cancer, or mediastinic masses), early satiety, nausea, vomiting (gastric and small bowel tumors), abdominal pain for intestinal sub-occlusion or occlusion (small and large bowel, peritoneal carcinomatosis). Moreover, the tumor can interfere with organ function, for example, causing diarrhea (pancreatic and biliary cancer) as a result of the lack of digestive enzymes [1, 11, 12]. The presence of continuous or occasional pain during eating and digestion may represent another important factor limiting, quantitatively and qualitatively, oral intake [6, 13].
Furthermore, following surgery for cancer removal, gastrointestinal changes can affect the digestive processes causing, for example, early satiety, dumping syndrome (gastric surgery), or diarrhea (pancreatic and colonic resection). Each condition requires specific dietetic interventions aimed at progressive nutritional rehabilitation. Finally, anticancer treatments (chemotherapy and radiation therapy) can cause anorexia, early satiety, nausea, vomiting, oral and intestinal mucositis with dysphagia, diarrhea, hemorroids, anal fissures, and modifications in smell and taste senses. All these symptoms may affect food choices and contribute to inadequate meal intake and reduced quality of life [1, 8]. In some cases, co-administered medications, taken to control symptoms or to treat adverse effects associated with anticancer treatments, can produce their own adverse events [6].
2.1 Interaction between malnutrition and therapy
The nutritional and inflammatory status appears to be correlated with an increased risk of severe hematological toxicity following anticancer chemotherapy [14]. Moreover, chemotherapy-induced DNA damage might become more severe in normal tissues in the presence of alterations of the cellular immune response due to high protein catabolism and stimulation of the acute-phase response [15]. Malnutrition can influence the outcome of chemotherapy, radiation, and surgery for cancer due to changes in metabolism, pharmacokinetics, and healing dynamics. Moreover, malnutrition could be responsible for alterations in absorption, protein binding, hepatic metabolism, and renal elimination of drugs and their metabolites [15, 16]. In malnourished patients, a reduced concentration of plasma proteins may significantly increase the likelihood of toxicity by agents with high protein binding, such as prednisolone, etoposide, cisplatinum, paclitaxel, and irinotecan metabolites [15].
Malnutrition decreases the oxidative metabolism in the liver performed by cytochrome P-450 isoenzymes through a depletion of nicotinamide adenine dinucleotide phosphate reserves. Other liver metabolic pathways can also be impaired, decreasing clearance and thus prolonging drug half-life. This may lead to greater drug exposure, which may influence toxicity [15]. The effect of malnutrition on renal function is less clear, but measurements of inulin clearance in malnourished children showed a reduced glomerular filtration rate [16].
2.2 Quality of life
The various complications caused by cancer can affect patients’ quality of life in many domains: physical (pain, sleep disturbances, loss of appetite, fatigue, activities of daily living), social, psychological (depression, anxiety), and work-related [17, 18]. Depression is a known factor in the pathophysiology of anorexia and other nutritional troubles [19]. Together with the nutritional intervention, a full and appropriate assessment of the patient’s needs must be performed. A baseline assessment also highlights current physical and emotional problems that the patient may be experiencing [17, 18].
3 Malnutrition and cachexia
Malnutrition is a clinical condition of imbalance of energy, protein, and other nutrients which causes measurable adverse effects on tissue/body composition, function, and clinical outcome [20, 21]. Anorexia and reduced food intake are frequently neglected in patients with cancer and contribute to nutritional impairment. Malnutrition, if not properly and early treated, unavoidably progresses to cachexia [22]. Not all malnourished patients are cachectic, but all cachectic patients are invariably malnourished [20].
Cachexia is a multifactorial syndrome characterized by severe body weight, fat and muscle loss, and increased protein catabolism due to underlying disease(s) as cancer, AIDS, chronic obstructive lung disease, and congestive heart failure. It results from a complex interplay between underlying disease, disease-related metabolic alterations, and reduced availability of nutrients (reduced intake, impaired absorption, and/or increased losses). Once established, cachexia cannot be reversed, neither nutritionally nor with other treatments, and increases patients’ morbidity and mortality. Contributory factors to the onset of cachexia are anorexia and metabolic alterations, i.e., inflammatory status, increased muscle proteolysis, impaired carbohydrate, and protein and lipid metabolism [20].
Inflammation plays a pivotal role in the pathogenesis of cachexia: an imbalance between pro-inflammatory (e.g., tumor necrosis factor-α [TNF-α], IL-1, IL-6, interferon-γ [IFN-γ]) and anti-inflammatory (e.g., IL-4, IL-12, IL-15) cytokines is currently considered to contribute to cachexia development and progression [23]. Pre-cachexia immediately precedes cachexia and can be diagnosed in the presence of all the following criteria: (a) underlying chronic disease; (b) unintentional weight loss >=5% of usual body weight during the last 6 months; (c) chronic or recurrent systemic inflammatory response; and (d) anorexia or anorexia-related symptoms.
Progressive loss of skeletal muscle mass is the most clinically relevant phenotypic feature of cachexia and causes negative clinical consequences on muscle strength, respiratory function, functional status, and quality of life [24]. Muscle loss, with increased muscle protein degradation results from an imbalance between anabolic and catabolic rates. Systemic inflammation is one of the main actors together with tumor-derived factors such as proteolysis-inducing factor, bed rest, and inadequate nutrient intake [24, 25].
4 Nutritional screening and nutritional assessment
Nutritional screening aims to identify patients at risk of malnutrition in a simple and non-invasive way. A full evaluation (nutritional assessment) to confirm and classify the degree of malnutrition will follow. These two steps allow to early identify and treat patients with malnutrition or periodically follow-up those at high nutritional risk, to face any nutritional challenges before significant weight loss or before other clinical/biological signs of malnutrition appear [26, 27].
Due to time restrictions by healthcare professionals, practical organization and costs, nutritional screening has necessarily to be feasible, speedy, inexpensive, and non-invasive.
Several more or less complex criteria have been proposed for nutritional assessment. The full evaluation of the patients should include data collection about:
-
1.
Social demography (gender, age, professional status, and living conditions)
-
2.
Primary tumor (site, disease stage at diagnosis) and the management of the disease (previous and on-going treatments)
-
3.
Anthropometry (actual weight, body mass index [BMI, the weight in kilograms divided by the height in centimeters squared], usual healthy body weight, unintentional weight loss since the start of the illness, or appearance of the first symptoms; weight loss during the last week, the last month, and over the last 6 months;
-
4.
Clinical examination, Subjective Global Assessment [28] and Karnofsky Index (evaluating functional capacity) [29];
-
5.
Biochemical data: serum albumin, prealbumin, total lymphocyte count cholesterol, C reactive protein (CRP), pseudocholinesterase (PChE) [30–32].
Careful clinical history record and meticulous symptom assessment are the first steps to become sympathetic with the patient and to understand his/her conditions in more detail. Another major step is the compilation, with the help of a dietitian, of a detailed food record in the days between chemotherapy cycles (when patients are feeling better) and the days of treatment; the compilation of questionnaires about patients’ daily activities (ability to work, social life, autonomy in personal hygiene, time spent in bed) should also be recommended [33].
A nutritional screening should be performed at the time of the diagnosis, possibly before starting the specific anticancer treatments. Therefore, for patients at risk of malnutrition, the clinical nutritionist evaluates the patient’s conditions and jointly with the dietitian, gives strategic advices on dietary intakes and choices, according to the scheduled treatment. If the patient is already malnourished or at high risk of malnutrition, a nutritional support should be promptly prescribed [34]. Independent of whether patient is or is not malnourished, periodical follow-up should be established and timed on the schedules of the oncologic therapies; an appropriate follow-up should be performed every 2 or 3 weeks [30–34].
4.1 Which nutritional parameter is the most reliable?
BMI alone is not a sensitive parameter to analyze nutritional status because it may still yield high or normal results in patients with ascites or edema. Unintentional weight loss could instead be the best single parameter for detecting malnutrition [30, 35]. Serum albumin level is the most widely used clinical nutritional index, but for its relatively long half-life and correlation with stress and illness, it remains a non-specific parameter of nutritional status. Indeed, pro-inflammatory tumor-derived mechanisms influence the acute-phase protein response in the liver and thus albumin synthesis. Acute-phase reactants comprise a range of proteins that rapidly change in plasma concentration responding to inflammation or tissue injury [36].
“Positive” acute-phase proteins, such as CRP, directly rise with inflammatory disease activity. “Negative” acute-phase proteins (albumin, prealbumin, and transferrin) respond inversely: their levels decrease in response to injury and inflammation and increase during recovery from the same conditions. Decreased serum levels of albumin, prealbumin, and transferrin do not necessarily reflect a state of malnutrition, but could be a consequence of the body’s physiologic response to injury. Under these conditions, resolution of inflammation, and not exogenous substrate (protein and energy) from nutritional support, restores normal hepatic protein metabolism and, eventually, serum levels of negative acute-phase proteins [36].
Recently, the role of plasma PChE as nutritional index has been described: its levels were found to be decreased in malnourished patients with or without hepatic involvement [37]. One of the possible mechanisms responsible for PChE activity reduction in cancer patients could be anorexia, which accompanies malignancy, as happens in anorexia nervosa too [38]. Bozzetti et al. observed that PChE, body weight, and nitrogen balance improved during nutritional support in cancer patients [39]. To adequately assess nutritional status in patients with cancer and in order to avoid possible interferences by inflammation and tumor-derived factors, not a single parameter but a cluster of two or more parameters should be considered [12, 35].
5 Treatment of malnutrition
5.1 Nutritional intervention and supplement
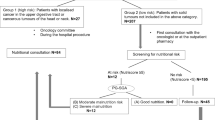
Research in clinical oncology has focused mainly on defining the best practice to evaluate anticancer treatment—cytotoxic drugs, radiation, and surgical procedures—as well as to clarify the traditional endpoints of disease-free and overall survival. Malnutrition is associated with negative outcomes including increased morbidity, poor prognosis and tolerance to treatment, decreased quality of life, and increased health care costs [1]. Patients with or at risk of malnutrition should receive the most appropriate nutritional support (oral supplements or enteral/parenteral support; Fig. 1). Furthermore, such patients should be followed-up during the evolution of the disease. For each single patient, for the specific type of cancer and for the involved body areas, all variables that could negatively affect the nutritional status should be identified and treated according to appropriate protocols and decisional algorithms [32].
The nutritional support has to be personalized according to the nutritional status of the single patient, to the toxicity of the respective patient’s therapy, and to the influence of symptoms on the daily eating requirements. For example, in case of nausea and vomiting in the 5–7 days following chemotherapy with strong impairment of eating in already malnourished patients, parenteral nutritional support can be considered. Under the same conditions, for a well-nourished patient, a hydrating–detoxifying therapy could be appropriate [34, 37].
Head/neck cancer patients generally experience a considerable weight loss and malnutrition for dysphagia, mucositis, and xerostomy during and following radiation therapy. Reported risk factors associated with serious weight loss are: baseline Karnofsky performance status less than 80, combination with chemotherapy, and receiving a total dose of radiation of 60 Gray or more [29]. Treatment toxicity may be exacerbated by poor nutritional status before and during therapy and may impair recovery time because of the effects of malnutrition on wound healing. Malnutrition may compromise treatment efficacy and reduce quality of life, possibly even affect survival [4, 7].
In patients with head/neck cancer on radiotherapy or combined chemo- and radiotherapy, weight loss generally commences in the second week of therapy. Considering that the gastrointestinal tract distal of the tumor and the treatment field is usually functional, enteral rather than parenteral feeding yields safer and more physiological results. The positioning of a nasogastric tube (NGT) for enteral nutrition can be advisable when a temporary dysphagia is anticipated (during and soon after radiation therapy). On the other hand, at stage 3 or 4 of the disease, the prophylactic placement of a percutaneous gastrostomy (PEG) for early enteral nutrition can be a reasonable approach [40].
While NGT positioning has generally been recommended when nutritional support is required for a short period of time (less than 1 month), PEG tubes are the better choice for prolonged nutritional support [41].
In malnourished patients who undergo radiotherapy as single or concomitant therapy for gastrointestinal neoplasms, abdominal pain, nausea, vomiting, or diarrhea may not permit an adequate oral support and intestinal absorption. Therefore, a parenteral nutritional therapy through peripheral veins or preferably through a pre-existing central venous catheter could be necessary [42].
In patients requiring abdominal radiation, enteritis may develop, causing malabsorption and/or obstruction due to intestinal fibrosis or stenosis. These patients need specific dietary recommendations, such as semiliquid, hyperproteic, fiber-free, or lactose-free diet [43, 44].
Adequate education regarding the prevention and treatment of diarrhea and constipation is also important. Dietary supplements and alternative foods have to be discussed and prescribed [45, 46]. Also, patients with a regular oral intake need to be followed-up closely to prevent or diagnose and treat malnutrition as early as possible.
During inflammatory processes, tissue depletion results in protein and fat loss as a result of the actions of pro-inflammatory cytokines. Besides the inflammatory condition of cancer, chemotherapy increases oxidative stress, thereby providing a further boost to the inflammatory process. Hypercaloric/hyperproteic oral supplements could be indicated, due to the hypercatabolism induced by the tumor and chemotherapy [47, 48].
Many formulas are now disposable, with high or low fat content, enriched with anti-inflammatory and antioxidant agents. Among nutrients acting on the inflammatory process are n-3-polyunsaturated fatty acids (n-3-PUFA) and antioxidants. Eicosapentaenoic acid (EPA) is an n-3, 21-carbon atom, polyunsaturated fatty acid, with five double bonds, found in oily fish. It seems to attenuate weight loss, particularly loss of skeletal muscle mass by down-regulating the increased expression and activity of the ubiquitin–proteasome proteolytic pathway [45, 47, 48]. Since EPA has no effects on protein synthesis in muscle, EPA has been combined with nutritional supplements rich in protein and energy, such as branched chain amino acids [49].
Fearon’s group has pioneered the use of fish oil in the treatment of pancreatic cancer, reporting the ability of n-3 PUFA, in particular, EPA and docosahexaenoic acid to reduce weight loss rate in such patients. Glutamine acts as a source of glutamate and may provide one of the three amino acids (glycine, cysteine, and glutamate) required for the synthesis of the key antioxidant glutathione, indirectly reducing inflammatory stress in the patients [45]. Antioxidants represent one of the largest categories of dietary supplements, which could be protective against the adverse effects of chemotherapy [49]. Severely malnourished patients with decreased dietary carnitine uptake may develop carnitine deficiency when treated with cisplatin, as the drug increases renal carnitine excretion approximately tenfold [50].
Trace elements consist mostly of metal ions acting mainly as basic components of essential enzymatic systems or proteins, which play major roles in the physiology of the gastrointestinal tract. Some studies suggest that trace elements serve as co-factors in several metabolic pathways and a decrease in their concentration may facilitate the malnutrition process. Selenium deficiency may interfere with free radical-mediated damage. Zinc regulates the function of cytochromes, stabilizes plasma membranes, reduces lipid peroxidation, and has a role in the detoxification of ammonia. Supplementation of these trace elements can delay cachexia onset with its subsequent depression of the immune system, influencing the neoplastic process and the effect of chemotherapy [51, 52].
5.2 Agents affecting appetite
Cachexia is strongly associated with anorexia. Besides oral caloric supplementation, early preventive and treatment attempts have thus suggested the use of appetite stimulants. The most widely prescribed is megestrol acetate, a synthetic progestin, which may stimulate appetite via neuropeptide Y in the ventromedial hypothalamus and by down-regulating the synthesis and release of pro-inflammatory cytokines. A systematic review of 15 randomized clinical trials of high-dose progestin therapy has showed a statistically significant improvement in both appetite and body weight. However, body composition analysis of patients who gained weight showed that weight gain was due to increased fat and not lean body mass [53].
Similar results have been obtained with another progestin, medroxyprogesterone acetate [54]. The inability of these two progestins to increase lean body mass would explain why patients show no significant improvement in the Karnovsky Index (performance score) or in quality of life. Moreover, attention should be paid on the prescription of progestins for the increased risk of thrombo-embolic phenomena and edema [55]. Thus, cyproheptadine, a histamine antagonist with antiserotonergic and appetite-stimulating effects, produces only a slight improvement in appetite and does not significantly prevent progressive weight loss in anorectic cancer patients [56].
Corticosteroids such as dexamethasone, prednisolone, and methylprednisolone are used to enhance appetite, sensation of well-being and performance, usually at the end-stage of cancer, because of their catabolic effect on skeletal muscle. However, despite possible improvement in quality of life, they have no other beneficial effects [57]. Several neuropeptides regulate appetite and are currently under evaluation to assess their efficacy in the treatment of cancer anorexia/cachexia. Among these is ghrelin, a neuropeptide released from the stomach in response to fasting that stimulates food intake. The use of ghrelin has recently been reviewed elsewhere [58].
5.3 Treatment of mucositis
The cytotoxic effects of chemotherapy and radiation therapy can cause an inflammatory response of mucosal epithelial cells called mucositis. All mucous membrane-covered surfaces, from mouth to rectum, may be affected [59]. Oral mucositis disrupts the function and integrity of the oral cavity, affecting a satisfying oral food intake and negatively influencing treatment outcomes and quality of life. It is associated with significant clinical morbidity, which may include pain, dysphagia, malnutrition, and local and systemic infections.
The largest damage at the oral mucosa is seen in patients with head/neck cancer treated with a combination of radio- and chemotherapy. Although present throughout the gastrointestinal tract, mucositis in the oral cavity has been better characterized in the literature because of the ease of assessment [60]. Oral care is widely considered the basis of mucosal health, integrity, and function. However, the specific components, methods, and frequency of this treatment remain in debate, partly because of the ethical considerations of withholding oral care in clinical trials [62].
In physiological conditions, the human saliva has lubricating properties and antibacterial/antiviral actions; disturbances of the salivary flow alter the protective role of oral microflora. Oral care protocols could help to minimize the effects of oral mucositis in patients receiving treatment for cancer: it can reduce the amount of microbial flora, control pain and bleeding, and prevent infection. Good oral health also reduces the risk of gingival and dental complications [58, 59]. Few data are available on the use of sodium-bicarbonate rinsing to alkalinize oral cavity, followed by the prophylactic use of an antiseptic (chlorexidine-based) and/or antifungine (nystatin, itraconazole) mouth washes. Also doubtful is the benefit of using local hydrating agents containing jaluronic acid and other cicatrizing agents.
6 Cancer rehabilitation
The clinical evaluation of patients with cancer has to include nutritional assessment [1, 28]. Depending on the needs of the patient and his/her family, members of the rehabilitation team may include any or all involved physicians, oncology nurses, dietitians, physiotherapists, and psychologists. The team has to address the potential rehabilitation needs of the individual from cancer diagnosis to rehabilitation, covering the following objectives: pain-killer therapy, nutritional and psychosocial support, and optimization of physical and social functioning [2, 8]. The implementation of a nutritional surveillance could enable rapid treatment of symptoms (anorexia, dysphagia, nausea, vomiting, constipation, tiredness, etc.), which could have a role in the malnutrition process [4]. The first step of cancer rehabilitation is the primary support to the negative effects of cancer disease and its therapy [6–8]. Oncologists have to be encouraged to share their work with other team specialists: anesthesiologists for pain therapy, nutritionists for nutritional support, and physical and rehabilitation medicine specialists. Physiotherapists, dietitians, and specialized nurses complete the team. All these figures have to cooperate to symptoms’ management of cancer patients [32, 33]. It is necessary to achieve early treatment of malnutrition to avoid a gradual progression to cachexia, an irreversible condition. Patients have to be treated when an effective intervention is still possible, before cachexia manifests. It will be meaningless and unethical to treat the irreversible phase of cachexia, when the patient is almost at the end of life [61, 62]. In that condition, hydration is indicated [63]. Malnutrition and above all cachexia have to be prevented and/or followed-up from the first diagnosis of cancer [64].
Evaluating data reported in the literature, it appears that few patients receive early nutritional counseling and nutritional issues are often underestimated in the diagnostic and therapeutic course of cancer rehabilitation [65]. This seems to be related to excessive workload and insufficient knowledge/skill among healthcare professionals expected to take on the task of screening.
7 Conclusions
It is necessary to make all healthcare professionals aware of the opportunity to screen cancer patients and identify those at risk of malnutrition. It is a duty of any physician and the patients’ right to improve preventive actions and intervene early during cancer treatment and progression of the disease [4, 6].
References
van Bokhorst-de van der Schueren MA. Nutritional support strategies for malnourished cancer patients. Eur J Oncol Nurs. 2005;9(Suppl 2):S74–83.
Fearon KC, Voss AC, Hustead DS, Cancer Cachexia Study Group. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–50.
von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:159–67.
Capra S, Ferguson M, Ried K. Cancer: impact of nutrition intervention outcome–nutrition issues for patients. Nutrition. 2001;17:769–72.
Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27:5–15.
Alexandre J, Gross-Goupil M, Falssard B, et al. Evaluation of the nutritional and inflammatory status in cancer patients for the risk assesment of severe haematological toxicity following chemotherapy. Ann Oncol. 2003;14:36–41.
Andreyev HJ et al. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–9.
Khalid U, Spiro A, Baldwin C, Sharma B, McGough C, Norman AR, et al. Symptoms and weight loss in patients with gastrointestinal and lung cancer at presentation. Support Care Cancer. 2007;15:39–46.
Gupta D, Lis CG, Vashi PG, Lammersfeld CA. Impact of improved nutritional status on survival in ovarian cancer. Support Care Cancer. 2010;18:373–81.
Skipworth RJE, Stewart GD, Dejong CHC, Preston T, Fearon KC. Pathophysiology of cancer cachexia: much more than host-tumor interaction? Clin Nutr. 2007;26:667–76.
Marín Caro MM, Laviano A, Pichard C. Nutritional intervention and quality of life in adult oncology patients. Clin Nutr. 2007;26:289–301.
Santarpia L, Alfonsi L, Pasanisi F, De Caprio C, Scalfi L, Contaldo F. Predictive factors of survival in patients with peritoneal carcinomatosis on home parenteral nutrition. Nutrition. 2006;22:355–60.
Gaidos JK, Gaidos JK, Draganov PV. Treatment of malignant gastric outlet obstruction with endoscopically placed self-expandable metal stents. World J Gastroenterol 2009;21; 15:4365–71.
Alexandre J, Gross-Goupil M, Falissard B, Nguyen ML, Gornet JM, Misset JL, et al. Evaluation of the nutritional and inflammatory status in cancer patients for the risk assessment of severe haematological toxicity following chemotherapy. Ann Oncol. 2003;14:36–41.
Murry D, Riva L, Poplack D. Impact of nutrition on pharmacokinetics of antineoplastic agents. Int J Cancer. 1998;S11:48–51.
Campbell KL, Ash S, Davies PS, Bauer JD. Randomized controlled trial of nutritional counseling on body composition and dietary intake in severe CKD. Am J Kidney Dis. 2008;51:748–58.
Das P, Cantor SB, Parker CL, Zampieri JB, Baschnagel A, Eng C, et al. Long-term quality of life after radiotherapy for the treatment of anal cancer. Cancer. 2010;116:822–9.
Ozturk A, Sarihan S, Ercan I, Karadag M. Evaluating quality of life and pulmonary function of long-term survivors of non-small cell lung cancer treated with radical or postoperative radiotherapy. Am J Clin Oncol. 2009;32:65–72.
Vilmar A, Santoni-Rugiu E, Sørensen JB. ERCC1, toxicity and quality of life in advanced NSCLC patients randomized in a large multicentre phase III trial. Eur J Cancer. 2010;46:1554–62.
Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–9.
Lochs H, Allison SP, Meier R, et al. Introductory to the ESPEN guidelines on enteral nutrition: terminology, definitions and general topics. Clin Nutr. 2006;25:180–6.
Braun TP, Marks DL. Pathophysiology and treatment of inflammatory anorexia in chronic disease. J Cachexia Sarcopenia Muscle. 2010;1:135–45.
Saini A, Al-Shanti N, Stewart CE. Waste management—cytokines, growth factors and cachexia. Cytokine Growth Factor Rev. 2006;17:475–86.
Costelli P, Baccino FM. Mechanisms of skeletal muscle depletion in wasting syndromes: role of ATP-ubiquitin-dependent proteolysis. Curr Opin Clin Nutr Metab Care. 2003;6:407–12.
Tisdale MJ. The “cancer cachectic factors”. Support Care Cancer. 2003;11:73–8.
Nourissat A, Vasson MP, Merrouche Y, Bouteloup C, Goutte M, Mille D, et al. Relationship between nutritional status and quality of life in patients with cancer. Eur J Cancer. 2008;44:1238–42.
Huhmann MB, Cunningham RS. Importance of nutritional screening in treatment of cancer-related weight loss. Lancet Oncol. 2005;6:334–43.
Barbosa-Silva MC. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11:248–54.
Batel-Copel LM, Kornblith AB, Batel PC, Holland JC. Do oncologists have an increasing interest in the quality of life of their patients? A literature review of the last 15 years. Eur J Cancer. 1997;33:29–32.
Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–21.
Duguet A et al. Summary report of the standards, options and recommendations for malnutrition and nutritional assessment in patients with cancer 1999. Br J Cancer 2003;89(Suppl. 1):S92–7, S92–S97.
Isenring E, Bauer J, Capra S. The scored Patient-generated Subjective Global Assessment (PG-SGA) and its association with quality of life in ambulatory patients receiving radiotherapy. Eur J Clin Nutr. 2003;57:305–9.
Ravasco P, Monteiro-Grillo I, Vidal PM, Camilo ME. Dietary counseling improves patient outcomes: a prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J Clin Oncol. 2005;23:1431–8.
Bozzetti F, Arends J, Lundholm K, Micklewright A, Zurcher G, Muscaritoli M, et al. ESPEN guidelines on parenteral nutrition: non-surgical oncology. Clin Nutr. 2009;28:445–54.
Santarpia L, Marra M, Montagnese C, Alfonsi L, Pasanisi F, Contaldo F. Prognostic significance of bioelectrical impedance phase angle in advanced cancer: preliminary observations. Nutrition. 2009;25:930–1.
Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104:1258–64.
Gu S-Z et al. Alterations of serum cholinesterase in patients with gastric cancer. World J Gastrenterol. 2005;11:4604–6.
Grandone L, Santarpia L, Alfonsi L, Pagano MC, Pasanisi F, Contaldo F. Serum cholinesterase as indicator of parenteral nutrition efficacy in protein energy malnutrition: four case reports. e-SPEN (The European e-Journal of Clinical Nutrition and Metabolism) - 18 November 2009 (10.1016/j.eclnm.2009.10.006).
Bozzetti F. Effects of artificial nutrition on the nutritional status of cancer patients. J Parenter Enteral Nutr. 1989;13:406–20.
Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral Nutrition during the treatment of head and neck carcinoma is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer. 2001;91:1785–90.
Cady J. Nutritional support during radiotherapy for head and neck cancer: the role of prophylactic feeding tube placement. Clin J Oncol Nurs. 2007;11:875–80.
Gunnlaugsson A, Kjellén E, Nilsson P, Bendahl PO, Willner J, Johnsson A. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol. 2007;46:937–44.
Gore JI, Surawicz C. Severe acute diarrhea. Gastroenterol Clin North Am. 2003;32:1249–67.
MacNaughton WK. Review article: new insights into the pathogenesis of radiation-induced intestinal dysfunction. Aliment Pharmacol Ther. 2000;14:523–8.
Fearon KC et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomized double blind trial. Gut. 2003;52:1479–86.
Linard C, Ropenga A, Vozenin-Brotons MC, Chapel A, Mathe D. Abdominal irradiation increases inflammatory cytokine expression and activates NF-kappaB in rat ileal muscularis layer. Am J Physiol Gastrointest Liver Physiol. 2003;285:G556–65.
Cerchietti LC, Navigante AH, Castro MA. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer. 2007;59:14–20.
Dewey A, Baughan C, Dean T, Higgins B, Johnson I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev. 2007;24:CD004597.
Siddiqui R, Pandya D, Harvey K, Zaloga GP. Nutrition modulation of cachexia/proteolysis. Nutr Clin Pract. 2006;21:155–67.
Heuberger W, Berardi S, Jacky E, et al. Increased urinary excretion of carnitine in patients treated with cisplatin. Eur J Clin Pharmacol. 1998;54:503–8.
Federico A, Iodice P, Federico P, et al. Effects of selenium and zinc supplementation on nutritional status in patients with cancer of digestive tract. Eur J Clin Nutr. 2001;55:293–7.
Schrag D, Chung KY, Flombaum C, Saltz L. Cetuximab therapy and symptomatic hypomagnesiemia. J Natl Cancer Inst. 2005;97:1221–4.
Maltoni M, Nanni O, Scarpi E, Rossi D, Serra P, Amadori D. High-dose progestins for the treatment of cancer anorexia-cachexia syndrome: a systematic review of randomised clinical trials. Ann Oncol. 2001;12:289–300.
Wood L, Palmer M, Hewitt J, Urtasun R, Bruera E, Rapp E, et al. Results of a phase III, double-blind, placebo-controlled trial of megestrol acetate modulation of P-glycoprotein -mediated drug resistance in the first-line management of small-cell lung carcinoma. Br J Cancer. 1998;77:627–31.
Morley JE. Orexigenic and anabolic agents. Clin Geriatr Med. 2002;18:853–66.
Jatoi A. Weight loss in patients with advanced cancer: effects, causes, and potential management. Curr Opin Support Palliat Care. 2008;2:45–8.
Quinn B, Potting CM, Stone R, Blijlevens NM, Fliedner M, Margulies A, et al. Guidelines for the assessment of oral mucositis in adult chemotherapy, radiotherapy and haematopoietic stem cell transplant patients. Eur J Cancer. 2008;44:61–72.
Akamizu T, Kangawa K. Ghrelin for cachexia. J Cachexia Sarcopenia Muscle. 2010;1:169–76.
Worthington HV, Clarkson JE, Eden OB. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2007;17:CD000978.
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820–31.
Contaldo F, Alfonsi L, Santarpia L, Pasanisi F. Artificial nutrition at the bioethic cross-road between treatment and basic health care. Clin Nutr. 2006;25:171–2.
Bozzetti F. Home total parenteral nutrition in incurable cancer patients: a therapy, a basic humane care or something in between? Clin Nutr. 2003;22:109–11.
Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–9.
Bozzetti F. The patient with incurable aphagic cancer: to feed or not to feed? Nutrition. 2001;17:676–7.
Violante G, Alfonsi L, Santarpia L, Cillis MC, Negro G, De Caprio C, et al. Adult home parenteral nutrition: a clinical evaluation after a 3-year experience in a Southern European centre. Eur J Clin Nutr. 2006;60:58–61.
von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.
Acknowledgments
All authors of this manuscript comply with the guidelines of ethical authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [66].
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Santarpia, L., Contaldo, F. & Pasanisi, F. Nutritional screening and early treatment of malnutrition in cancer patients. J Cachexia Sarcopenia Muscle 2, 27–35 (2011). https://doi.org/10.1007/s13539-011-0022-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13539-011-0022-x