Abstract
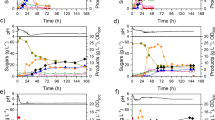
In this study, an attempt is made to optimize the effect of various physical and cultural parameters on butanol production by microbial strain Clostridium acetobutylicum MTCC 481 by employing L18 orthogonal array design of experiments. A set of five parameters, viz., temperature, pH, inoculum size, inoculum age, and agitation have been studied. Utilizing a pre-optimized rice straw hydrolysate medium, the clostridial strain produced maximum amount of butanol at optimum conditions of temperature 37 °C, pH 4.0 ± 0.5, inoculum size 5 % (v/v), inoculum age 18 h, and agitation 150 rpm. Among these parameters, pH, temperature, and agitation were found to be the most significant factors affecting solvent production. The optimized physical and cultural parameters were further verified at shake flask and bioreactor scale (2 L and 5 L bioreactor). Experiments using 2 and 5 L bioreactor under the optimized process condition showed nearly complete utilization of soluble sugars with the production of 15.84 g L−1 of total solvents with 12.17 g L−1 of butanol in 2 L bioreactor and 16.91 g L−1 of total solvents with 12.22 g L−1 of butanol in a 5 L of bioreactor, respectively. The experimental data were further validated by fitting it to a kinetic model reported in literature to determine the kinetic parameters of the fermentation process.






Similar content being viewed by others
Abbreviations
- ABE:
-
Acetone butanol ethanol
- ANOVA:
-
Analysis of variance
- CMM:
-
Cooked meat medium
- DNS:
-
Dinitrosalicylic acid
- DOE:
-
Design of experiments
- μ :
-
Specific growth rate (per hour)
- MS:
-
Mean of squares
- MTCC:
-
Microbial Type Culture Collection
- NCIM:
-
National Collection of Industrial Micro-organisms
- P :
-
Product concentration (grams per cubic liter)
- P 0 :
-
Initial product concentration (grams per cubic liter)
- P max :
-
Maximum product concentration (grams per cubic liter)
- P t :
-
Kinetic constant
- PABA:
-
p-Aminobenzoic acid
- RCA:
-
Reinforced clostridial agar
- RCM:
-
Reinforced clostridial medium
- RMSD:
-
Root mean square deviation
- RMSE:
-
Root mean square error
- RS:
-
Rice straw
- RSH:
-
Rice straw hydrolysate
- SS:
-
Sum of squares
- X :
-
Biomass concentration (grams per cubic liter)
- X m :
-
Maximum biomass concentration (grams per cubic liter)
- X 0 :
-
Biomass concentration (grams per cubic liter)
- Y P/S :
-
Product yield on the utilized substrate
- Y X/S :
-
Biomass yield on the utilized substrate
References
Cascone R (2008) Biobutanol—a replacement for bioethanol. Chem Eng Prog 104:S4–S9
Song H, Eom MH, Lee S, Lee J, Cho JH, Seung D (2010) Modeling of batch experimental kinetics and application to fed-batch fermentation of Clostridium tyrobutyricum for enhanced butyric acid production. Biochem Eng J 53:71–76
Tran HTM, Cheirsilpa B, Hodgsonb B, Umsakulc K (2010) Potential use of Bacillus subtilis in a co-culture with Clostridium butylicum for acetone–butanol–ethanol production from cassava starch. Biochem Eng J 48:260–267
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by clostridia. Biotechnol Bioeng 101:209–228
Ranjan A, Moholkar VS (2012) Biobutanol: science, engineering and economics. Int J Energ Res 39:277–323
Schmidt FR (2005) Optimization and scale up of industrial fermentation processes. Appl Microbiol Biotechnol 68:425–435
Leal MRLV, Walter AS, Seabra JEA (2012) Sugarcane as an energy source Biomass Conv. Bioref. doi:10.1007/s13399-012-0055-1
Machado de Castro S, Machado de Castro A (2012) Assessment of the Brazilian potential for the production of enzymes for biofuels from agroindustrial materials. Biomass Conv Bioref 2:87–107
Joensen F, Nielsen PEH, Sørensen MDP (2011) Biomass to green gasoline and power. Biomass Conv Bioref 1:85–90
Surisetty VR, Kozinski J, Dalai AK (2012) Biomass, availability in Canada, and gasification: an overview. Biomass Conv Bioref 2:73–85
Lenihan P, Orozco A, O’Neill O, Ahmad MNM, Rooney DW, Walker GM (2010) Dilute acid hydrolysis of lignocellulosic biomass. Chem Eng J 15:395–403
Orozco A, Ahmad M, Rooney D, Walker G (2007) Dilute acid hydrolysis of cellulose and cellulosic bio-waste using a microwave reactor system. Proc Safety Environ Protection 85:446–449
Cheng L, Keener TC, Lee JY, Zhou X (2012) Dilute acid pretreatment for cellulosic alcohol production. Biomass Conv Bioref 2:169–177
Manzanares P, Ballesteros I, Negro MJ, Oliva JM, Gonzalez A, Ballesteros M (2012) Biological conversion of forage sorghum biomass to ethanol by steam explosion pretreatment and simultaneous hydrolysis and fermentation at high solid content. Biomass Conv Bioref 2:123–132
Chiaramonti D, Rizzo AM, Prussi M, Tedeschi S, Zimbardi F, Braccio G, Viola E, Pardelli PT (2011) 2nd generation lignocellulosic bioethanol: is torrefaction a possible approach to biomass pretreatment? Biomass Conv Bioref 1:9–15
Thiry M, Cingolani D (2002) Optimizing scale-up fermentation processes. Trends Biotechnol 20:103–105
Stanbury PF, Whitakar A, Hall SJ (1997) Principles of fermentation technology. Aditya Books, New Delhi
Panda BP, Ali M, Javed S (2007) Fermentation process optimization. Res J Microbiol 2:201–208
Elizalde-González MP, García-Díaz LE (2010) Application of a Taguchi L16 orthogonal array for optimizing the removal of Acid Orange 8 using carbon with a low specific surface area. Chem Eng J 163:55–61
García IG, Martín AM, Ruiz JMO, Pérez AC (1989) Kinetic study of the production of ethanol with Saccharomyces cerevisiae immobilized on Berl saddles. Chem Eng J 42:B1–B7
Dasu VV, Panda T, Chidambaram M (2003) Determination of significant parameters for improved griseofulvin production in a batch bioreactor by Taguchi’s method. Proc Biochem 38:877–880
Chang MY, Tsai GJ, Houng JY (2006) Optimization of the medium composition for the submerged culture of Ganoderma lucidum by Taguchi array design and steepest ascent method. Enz Microb Tech 38:407–414
Oskouie SFG, Tabandeh F, Yakhchali B, Eftekhar F (2007) Enhancement of alkaline protease production by Bacillus clausii using Taguchi experimental design. Afr J Biotechnol 6:2559–2564
Wu X, Yang H, Guo L (2010) Effect of operation parameters on anaerobic fermentation using cow dung as a source of microorganisms. Int J Hyd Energ 35:46–51
Sanjari M, Taheri AK, Movahedi MR (2009) An optimization method for radial forging process using ANN and Taguchi method. Int J Adv Manuf Tech 40:776–784
Hedge JE, Hofreiter BT (1962) In Whistler RL, Miller JN (ed.) Carbohydrate chemistry. Academic Press, New York, p 17
Miller GL (1972) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Palaniraj RI, Nagarajan P (2012) Statistical analysis of experimental variables for the production of lactic acid using Lactobacillus casei from waste potato starch by Box-Behnken design. Int J Chem Tech Res 4:1049–1064
Bowles LK, Ellefson WL (1985) Effects of butanol on Clostridium acetobutylicum. Appl Environ Microbiol 50:1165
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50:484–524
Khamaiseh EI, Kalil MS, Dada O, El-Shawabkeh I, Yusoff WMW (1012) Date fruit as carbon source in RCM-modified medium to produce biobutanol by Clostridium acetobutylicum NCIMB 13357. J Appl Sci 12:1160–1165
Boon-Long S, Laguerie C, Coudere JP (1978) Mass transfer from suspended solids to a liquid in agitated vessels. Chem Eng Sci 33:813
Venil CK, Lakshmanaperumalsamy P (2009) Taguchi experimental design for medium optimization for enhanced production by Bacillus subtilis HB04. eJST 4:1–10
Sirisansaneeyakul S, Luangpipat T, Vanichsriratana W, Srinophakum T, Chen HHH, Chisti Y (2007) Optimization of lactic acid production by immobilized Lactococcus lactis IO-1. J Ind Microbiol Biotechnol 34:381–391
Marchal R, Blanchet D, Vandecasteele JP (1985) Industrial optimization of acetone-butanol fermentation: a study of the utilization of Jerusalem artichokes. Appl Microbiol Biotechnol 23:92–98
Marchal R, Ropars M, Pourqui J, Fayolle E, Vandecasteele JE (1992) Large-scale enzymatic hydrolysis of agricultural lignocellulosic biomass. part 2: conversion into acetone-butanol. Bioresourc Technol 42:205–217
Badr HR, Hamdy MK (1992) Optimization of acetone-butanol production using response surface methodology. Biomass Bioenerg 3:49–55
Syed Q, Nadeem M, Nelofer R (2008) Enhanced butanol production by mutant strains of Clostridium acetobutylicum in molasses medium. Turk J Biochem 33:25–30
Jieun L, Sen K, Kweon D, Park K, Jin Y (2009) Fermentation of rice bran and defatted rice bran for butanol production using Clostridium beijrinckii ncimb 8052. J Microbial Biotechnol 19:482–490
Marianoa AP, Costaa CBB, Angelisc DF, de Filhob FM, Atalab DIP, Maciela MRW, Filho RM (2010) Optimisation of a continuous flash fermentation for butanol production using the response surface methodology. Chem Eng Res Design 88:562–571
Liu Z, Li YYF, Ma C, Xu P (2010) Butanol production by Clostridium beijerinckii ATCC 55025 from wheat bran. J Ind Microbiol Biotechnol 37:495–501
Moon C, Lee CH, Sang B, Um Y (2011) Optimization of medium compositions favoring butanol and 1,3-propanediol production from glycerol by Clostridium pasteurianum. Bioresourc Technol 102:10561–10568
Wang Y, Blaschek HP (2011) Optimization of butanol production from tropical maize stalk juice by fermentation with Clostridium beijerinckii NCIMB 8052. Bioresourc Technol 102:9985–9990
Wang L, Chen H (2011) Increased fermentability of enzymatically hydrolyzed steam-exploded corn stover for butanol production by removal of fermentation inhibitors. Proc Biochem 46:604–607
Isar J, Rangaswamy V (2012) Improved n-butanol production by solvent tolerant Clostridium beijerinckii. Biomass Bioenerg 37:9–15
Lin Y, Wang J, Wang X, Sun X (2011) Optimization of butanol production from corn straw hydrolysate by Clostridium acetobutylicum using response surface method. Chinese Sci Bulletin 56:1422–1428
Tran HTM, Cheirsilp B, Umsakul K, Bourtoom T (2011) Response surface optimisation for acetone-butanol-ethanol production from cassava starch by co-culture of Clostridium butylicum and Bacillus subtilis. Maejo Int J Sci Technol 5:374–389
Ranjan A, Moholkar VS (2011) Comparative study of various pretreatment techniques for rice straw saccharification for the production of alcoholic biofuels. Fuel doi:10.1016/j.fuel.2011.03.030.
Mercier P, Yerushalmi L, Rouleau D, Dochain D (1992) Kinetics of lactic acid fermentation on glucose and corn by Lactobacillus amylophilus. J Chem Tech Biotech 55:111–121
Rodrigues L, Moldes A, Teixeira J, Oliveira R (2006) Kinetic study of fermentative biosurfactant production by Lactobacillus strains. Biochem Eng J 28:109–116
Cheng C, Che P, Chen B, Lee W, Chien LJ, Chang JS (2012) High yield bio-butanol production by solvent-producing bacterial microflora. Bioresourc Technol 113:58–64
Acknowledgment
The authors acknowledge the Ministry of New and Renewable Energy for providing NRE fellowship to Ms. Amrita Ranjan. The infrastructural and analytical facilities provided by Centre for Energy and Department of Chemical Engineering, IIT Guwahati, and Spectrophotometric analysis facility provided by CIF, IIT Guwahati, are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ranjan, A., Mayank, R. & Moholkar, V.S. Process optimization for butanol production from developed rice straw hydrolysate using Clostridium acetobutylicum MTCC 481 strain. Biomass Conv. Bioref. 3, 143–155 (2013). https://doi.org/10.1007/s13399-012-0062-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-012-0062-2