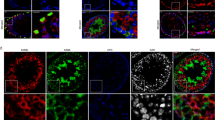
Abstract
Nanotechnology has been increasingly utilized for the targeting and delivery of novel therapeutic agents to different tissues and cell types. The current therapeutic options for testicular disorders fall short in many instances due to difficulty traversing the blood–testis barrier, systemic toxicities, and complicated dosing regiments. For testicular tissue, potential targeting can be obtained either via anatomic methods or specific ligands such as luteinizing hormone or follicle-stimulating hormone analogs. Potential novel therapeutic agents include DNA, RNA, cytokines, peptide receptor antagonists, peptide receptor agonists, hormones, and enzymes. Nanotherapeutic treatment of testicular cancer, infertility, testicular torsion, orchalgia, hypogonadism, testicular infections, and cryptorchidism within the framework of potential target cells are an emerging area of research. While there are many potential applications of nanotechnology in drug delivery to the testis, this remains a relatively unexplored field. This review highlights the current status as well as potential future of nanotechnology in the development of novel therapeutics for testicular disorders.



Similar content being viewed by others
References
Rugendorff EW. Patent medicines for the treatment of genitourinary disease. Word J Urol. 1999;17:171–5.
Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release. 2011;152:330–48.
Witkin SS, Jeremias J, Bongiovanni AM, Munoz MG. Immune regulation in the male genital tract. Infect Dis Obstet Gynecol. 1996;4(3):131–5.
Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol. 2003;170(1):5–11.
Granitsiotis P, Kirk D. Chronic testicular pain: an overview. Eur Urol. 2004;45(4):430–6.
Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–12.
Povey AC, Stocks SJ. Epidemiology and trends in male subfertility. Hum Fertil. 2010;13(4):182–8.
Lee KC. Fertility treatments and the cost of a healthy baby. Nurs Wom Health. 2011;15(1):15–8.
Richie JP, Steele GS. Neoplasms of the testis. In: Kavoussi LR, Novick AC, Partin AW, Peters CA, Wein AJ, editors. Campbell's Urology. 9th ed. Saunders Elsevier; 2007. pp. 893–935.
Sigman M, Jarow JP. Male infertility. In: Kavoussi LR, Novick AC, Partin AW, Peters CA, Wein AJ, editors. Campbell's Urology. 9th ed. Saunders Elsevier; 2007. pp. 609–653.
Hart SL. Multifunctional nanocomplexes for gene transfer and gene therapy. Cell Biol Toxicol. 2010;26(1):69–81.
Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8(24):1112–20.
Budhian A, Siegel SJ, Winey KI. Controlling the in vitro release profiles for a system of haloperidol-loaded PLGA nanoparticles. Int J Pharm. 2008;346(1–2):151–9.
Verma A, Stellacci F. Small. Effect of surface properties on nanoparticle-cell interactions. 2010;6(1):12–21.
Vasir JK, Reddy MK, Labhasetwar VD. Nanosytems in drug targeting: opportunities and challenges. Curr Nanosci. 2005;1(1):47–64.
Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–3.
Heneweer C, Holland JP, Divilov V, Carlin S, Lewis JS. Magnitude of enhanced permeability and retention effect in tumors with different phenotypes: 89Zr-albumin as a model system. J Nucl Med. 2011;52(4):625–33.
Brooks, JD. Anatomy of the lower urinary tract and male genitalia. In: Kavoussi LR, Novick AC, Partin AW, Peters CA, Wein AJ, editors. Campbell's Urology. 9th ed. Saunders Elsevier; 2007. pp. 38–80.
Sharpe RM. Paracrine control of the testis. Clin Endocrinol Metab. 1986;15(1):185–207.
Siu MK, Cheng CY. Extracellular matrix: recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biol Reprod. 2004;71(2):375–91.
Johnson L, Thompson Jr DL, Varner DD. Role of sertoil cell number and function on regulation of spermatogenesis. Anim Reprod Sci. 2008;105(1–2):23–51.
Maekawa M, Kamimura K, Nagano T. Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol. 1996;59(1):1–13.
Kerr JB. Functional cytology of the human testis. Baillieres Clin Endocrinol Metab. 1992;6(2):235–50.
Ghinea N, Milgrom E. A new function for the LH/CG receptor: transcytosis of hormone across the endothelial barrier in target organs. Semin Reprod Med. 2001;19(1):97–101.
Vu Hai MT, Lescop P, Loosfelt H, Ghinea N. Receptor-mediated transcytosis of follicle-stimulating hormone through the rat testicular microvasculature. Biol Cell. 2004;96(2):133–44.
Bhaskaran RS, Ascoli M. The post-endocytotic fate of the gonadotropin receptors is an important determinant of the desensitization of gonadotropin responses. 2005;34(2):447–57.
Krishnamurthy H, Kishi H, Shi M, Galet C, Bhaskaran RS, Hirakawa T, et al. Postendocytotic trafficking of the follicle-stimulating hormone (FSH)-FSH receptor complex. Mol Endocrinol. 2003;17(11):2162–76.
Segretain D, Gilleron J, Carette D, Denizot JP, Pointis G. Differential time course of FSH/FSH receptor complex endocytosis within Sertoli and germ cells during rat testis development. Dev Dyn. 2010;239(4):1113–23.
Thomas UM. Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol. 2005;18:S61–79.
Braydich-Stolle LK, Lucas BM, Schrand A, Murdock RC, Lee T, Schlager JJ, et al. Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol Sci. 2010;116(2):577–89.
Douglas ML, Richardson MM, Nicol DL. Testicular germ cell tumors exhibit evidence of hormone dependence. Int J Cancer. 2006;118(1):98–102.
Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363(17):1621–30.
Zhang XY, Chen J, Zhen YF, Gao XL, Kang Y, Liu JC, et al. Follicle-stimulating hormone peptide can facilitate paclitaxel nanoparticles to target ovarian carcinoma in vivo. Cancer Res. 2009;69(16):6506–14.
Shubayev VI, Pisanic TR, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61(6):467–77.
Lee NP, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development? Hum Reprod Update. 2004;10(4):349–69.
Lui WY, Lee WM. Regulation of junction dynamics in the testis-transcriptional and post-translational regulations of cell junction proteins. Mol Cell Endocrinol. 2006;250(1–2):25–35.
Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocrinol Rev. 2004;25(5):747–806.
Okanlawon A, Dym M. Effect of chloroquine on the formation of tight junctions in cultured immature rat sertoli cells. J Androl. 1996;17(3):249–55.
Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60(2):146–80.
Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. J Androl. 2008;29(5):488–98.
Turner TT, Tung KS, Tomomasa H, Wilson LW. Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod. 1997;57(6):1267–74.
Bal R, Turk G, Tuzcu G, Tuzcu M, Yilmaz O, Ozercan I, et al. Protective effects of nanostructures of hydrated C60 fullerene on reproductive function in streptozotocin-diabetic male rats. Toxicology. 2011;282:69–81.
Anderson JB, Williamson RC. Testicular torsion in Bristol: a 25-year review. Br J Surg. 1988;75(10):988–92.
Visser AJ, Heyns CF. Testicular function after torsion of the spermatic cord. BJU Int. 2003;92(3):200–3.
Tremellen K. Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update. 2008;14(3):243–58.
Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207(Pt 18):3221–31.
Comhaire F, Decleer W. Quantifying the effectiveness and cost-efficiency of food supplementation with antioxidants for male infertility. Reprod Biomed Online. 2011; doi:10.1016/j.rbmo.2011.05.009
Pinsky MR, Hellstrom WJ. Hypogonadism, ADAM and hormone replacement. Ther Adv Urol. 2010;2(3):99–104.
Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–31.
Ghosn J, Chaix ML, Peytavin G, Rey E, Bresson JL, Goujard C, et al. Penetration of enfuvirtide, tenofovir, efavirenz, and protease inhibitors in the genital tract of HIV-1 infected men. AIDS. 2004;18(14):1958–61.
Bay K, Main KM, Toppari J, Skakkebaek NE. Testicular descent: INSL3, testosterone, genes and the intrauterine milieu. Nat Rev Urol. 2011;8(4):187–96.
Acknowledgments
Further research in drug delivery for testicular disorders is made possible by a grant from the Cleveland Clinic Foundation Research Program Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Snow-Lisy, D.C., Samplaski, M.K., Labhasetwar, V. et al. Drug delivery to the testis: current status and potential pathways for the development of novel therapeutics. Drug Deliv. and Transl. Res. 1, 351–360 (2011). https://doi.org/10.1007/s13346-011-0039-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-011-0039-x