Summary
Spinal cord injury (SCI) is a devastating condition that affects approximately 11,000 patients each year in the United States. Although a significant amount of research has been conducted to clarify the pathophysiology of SCI, there are limited therapeutic interventions that are currently available in the clinic. Moderate hypothermia has been used in a variety of experimental and clinical situations to target several neurological disorders, including traumatic brain and SCI. Recent studies using clinically relevant animal models of SCI have reported the efficacy of therapeutic hypothermia (TH) in terms of promoting long-term behavioral improvement and reducing histopathological damage. In addition, several clinical studies have demonstrated encouraging evidence for the use of TH in patients with a severe cervical spinal cord injury. Moderate hypothermia (33°C) introduced systemically by intravascular cooling strategies appears to be safe and provides some improvement of long-term recovery of function. TH remains an experimental clinical approach and randomized multicenter trials are needed to critically evaluate this potentially exciting therapeutic intervention targeting this patient population.
Similar content being viewed by others
INTRODUCTION
Spinal cord injury is a devastating neurological disorder that affects both civilian and military personnel. Each year in the United States, approximately 11,000 to 12,000 individuals sustain a spinal cord injury from motor vehicle accidents, sport related injuries and direct trauma [1]. Most victims are young men, and the majority of these individuals are left with severe paralysis and functional deficits that remain for the rest of their lives. Through improvements in surgical procedures, stabilization approaches and critical care initiates, these individuals live relatively long lives with these devastating disabilities. Currently, there are no proven treatments that protect against the consequences of SCI [2–4], although methylprednisolone is used with some success for specific indications, according to the National Acute Spinal Cord Injury Study II protocol [5, 6]. Nevertheless, because of the medical costs associated with SCI and lack of effective treatments, there is a continued need to evaluate novel therapeutic interventions that can be initiated in the acute injury setting to limit secondary injury mechanisms and improve functional outcome in this patient population.
Hypothermia has been studied for many years and is found to be beneficial in a variety of acute CNS injuries [7–10]. In the early 1950s, profound levels of hypothermia were used in cardiac surgical procedures, as well as other acute indications, including stroke, brain trauma, and SCI [11–19]. These studies provided mixed results and cooling strategies were mostly abandoned when pharmacological agents were discovered that were believed to be neuroprotective.
However, more recently, the beneficial effects of more modest levels of hypothermia has been appreciated [7, 20–25]. In the 1980s, studies showed for the first time that relatively small reductions in brain tissue temperature provided significant protection against ischemic and traumatic neuronal cell death [7]. These studies led to a revival in the interest in the potential use of moderate hypothermia in a variety of experimental paradigms, including global and focal ischemia, cardiac arrest, traumatic brain injury, and SCI [7]. Therapeutic hypothermia has now gained acceptance primarily in treating patients with in-hospital cardiac arrest and babies experiencing hypoxic-insults during delivery [23]. Indeed, therapeutic hypothermia has recently been adopted by the American Heart Association as a treatment for cardiac arrest, and to date it is the only cytoprotective treatment that has been successfully translated from the bench to the bedside.
In terms of SCI, published data have shown that relatively mild levels of hypothermia introduced after a traumatic or compressive SCI provides some degree of improvement in function and reduces the histopathological damage [22, 25–37]. In clinically relevant SCI studies, mild reductions in temperature have been shown to be protective, whereas mild elevations (hyperthermia) have been reported to worsen outcome [38–40]. These studies have emphasized the importance of spinal cord temperature as an important factor in determining irreversible damage and severe neurological deficit. Importantly, moderate hypothermia induced systemically is protective in a variety of SCI models by many investigators and therefore merits consideration for clinical application.
Recently, case reports and clinical studies have provided encouraging results in terms of the safety and efficacy of moderate hypothermia following severe SCI [21]. In one high profile case, a professional football player who underwent severe cervical SCI by impact injury was treated with therapeutic hypothermia [41]. That individual did relatively well, in terms of long-term outcome, and generated significant interest in the use of hypothermia in the research and clinical community [21, 42]. Studies in a large series of SCI patients, initiated in 2005, showed that early cooling introduced by the use of endovascular catheters and continued for a 48-h period appeared to be safe and did not result in an increased incidence of risk factors, including cardiac arrhythmias and severe infection [43]. Most recently, the 1-year follow-up of these SCI patients showed an encouraging trend for improvement in function compared with an historical group of patients that were not cooled [44]. The purpose of this report is to summarize evidence for the use of therapeutic hypothermia in cases of severe SCI and provide a framework for future investigations.
HISTORICAL PERSPECTIVE
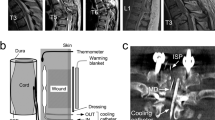
Therapeutic hypothermia has been investigated as a treatment strategy in various early clinical SCI studies [8]. In the 1960s, local profound hypothermia was produced in some patients by administering cold saline to the exposed spinal cord after laminectomy and during decompression surgeries [45, 46]. These studies, along with experimental observations, provided important information regarding the ability to cool locally, and in some cases resulted in functional improvement [19, 27, 47–49]. However, the interpretation of these studies was complicated by the fact that surgical interventions, including decompression procedures, may have led to some of the beneficial effects that were reported [46]. Additionally, the use of methylprednisolone as a protective steroid therapy in the acute injury setting was also a possible confounding issue [50, 51]. Another early obstacle in terms of the use of hypothermia was the problem of introducing systemic hypothermia to patients. As temperature began to decrease, shivering responses were noted as an attempt for the body to fight against the lowering temperatures [52, 53]. Different approaches to cooling included the use of cold fluids or ice baths, as well as externally placed cooling blankets, to reduce the temperature. These approaches were very cumbersome, and it was different to maintain critical levels of hypothermia for long periods of time.
In addition to trauma, various levels of hypothermia have also been shown to protect against periods of ischemia that may occur during transient periods of spinal cord compression or aortic reconstruction surgery [54–63]. In models of compression injury, hypothermia has been shown to improve neurological outcome, recovery of somatosensory evoked potentials and normal motor function [36, 64]. In studies in which the aorta is clamped for a period of time, hypothermia by epidural perfusion or other strategies of regional and systemic hypothermia have shown some promise in terms of reducing neurological deficits produced by the resulting spinal cord ischemia [65, 66]. In contrast to systemic hypothermia, local cooling allows very low levels of hypothermia to be introduced without potentially initiating inherent physiological effects, such as hypotension, bradycardia, and respiratory infection that can be seen in conditions in which systemic hypothermia is used [48, 52, 67, 68]. Nevertheless, a weakness of local cooling is that the procedure cannot be initiated until rather invasive surgical approaches are completed to allow for the application of cold fluid onto the surface of the injured spinal cord. The realization that only relatively moderate levels of hypothermia are required to produce improved outcome has allowed for systemic hypothermia to be evaluated in clinically relevant animal models, as well as targeted patient populations [7, 23, 43]. As previously mentioned, in the late 1960s and early 1970s, the interest in clinical hypothermia to treat acute neurological disorders had decreased due to the introduction of new pharmacological agents that could potentially provide similar neuroprotective results. More recent information has emerged that emphasizes the complexity of the pathophysiology of SCI, and the need for combination approaches or the use of “dirty drugs” to target multiple injury mechanisms has surfaced.
EXPERIMENTAL STUDIES
As previously described, many experimental studies in SCI have reported beneficial effects of either focal or systemic hypothermia [8, 20]. Martinez-Arizala and Green showed that both pre- and post-treatment with hypothermia (31–32°C) appeared to be effective in reducing the degree of hemorrhage at the site of SCI [24]. In other studies, more moderate degrees of systemic hypothermia have also been shown to promote motor recovery in both thoracic and cervical SCI models [7, 25]. In one study by Yu et al. [25], a whole-body moderate hypothermia (33°C) initiated by blowing cool air onto the surface of the rat led to significantly improved locomotive function, as assessed by the Basso, Beattie and Bresnahan (BBB) [69] open-field scoring system. In that study, hypothermia treatment was initiated 30 minutes after the injury and continued for a 4-h period. Following the hypothermic period, animals were slowly rewarmed and behaviorally tested for a 6-week duration. At the end of behavioral testing, perfusion-fixation was carried out and quantitative assessment of lesion volume was conducted. Importantly, the improved behavioral recovery was correlated with a significant reduction in both gray and white matter pathology (FIG. 1). This study showed for the first time that a moderate level of hypothermia initiated after the traumatic insult could improve both behavioral and histological outcome. In other studies, hypothermia protection has also been shown in models of compression injury that lead to reduced blood flow to the focal area of the injured spinal cord [26, 32, 35–37, 64]. In these studies, different levels or durations in cooling have also been shown in most cases to promote recovery. In a recent study by Batchelor et al. [26], the beneficial effect of hypothermia in decompressive SCI was assessed. In this study, decompression of the spinal cord was reduced by a spacer inserted to compress the spinal cord by 45%. In animals in which hypothermia was introduced prior to removal of the spacer, significant improvement in behavioral and histopathological outcomes was seen compared to normothermic animals. These investigators concluded that hypothermia may be useful as bridging therapy to prevent neurological decline prior to decompressive surgery.
Graph showing time course of locomotor recovery as measured by Basso, Beattie and Bresnahan (BBB) scores following hypothermic and normothermic treatment. Mean BBB scores obtained in animals receiving modest hypothermia (32–33°C) 30 minutes after trauma for 4 h are represented by the filled circles, and normothermia (37°C) are represented by triangles. Data are presented as mean ± standard error of the mean. *p < 0.05; **p < 0.01. (Reprinted with permission from Yu et al. [25]).
Many spinal cord injured patients sustain injuries at the cervical level. In an attempt to determine whether therapeutic hypothermia would help following severe cervical SCI, an animal model of SCI was developed [70]. In a study by Lo et al. [22], moderate hypothermia introduced after cervical SCI again led to improved behavioral and histopathological outcomes. Following cervical trauma, hypothermia was introduced by reducing the core temperature to 33° for a 4-h period followed by slow rewarming. Behavioral assessment that specifically determined the effects of cooling on hand function, as well as gait and lower motor function, showed that the hypothermic group improved better than that seen in the normothermic (37°C) animals. In addition, quantitative assessment of contusion volume demonstrated that the hypothermic group had smaller contusion areas. Most importantly, when a numbers of motor neurons were counted in the cervical gray matter area, hypothermic animals showed a greater preservation of motor neurons (FIG. 2). Taken together, these preclinical studies emphasize the beneficial effects of moderate hypothermia introduced after an ischemic or traumatic insult in terms of improving long-term outcome.
Hypothermia increased the numbers of preserved ventral motor neurons rostral and caudal to the injury site. Counts of cells labeled for NeuN (a neuron-specific marker) from transverse sections rostral (R) and caudal (C) to and within the injury epicenter of the cervical cord showed that acute application of mild systemic hypothermia could significantly increase the numbers of NeuN-immunoreactive neurons in the ventral horn (laminae VII–IX) at distances of 900 μm and greater from the injury epicenter compared with normothermic controls. Almost no preserved ventral motor neurons, however, were detected within the immediate injury site in both spinal cord injury (SCI) groups. Recordings from uninjured controls are provided for comparison, and the data are expressed as the average ± standard error of the mean. **p < 0.01 compared with normothermic controls; ***p < 0.001. (Reprinted with permission from Lo et al. [22]).
MECHANISMS UNDERLYING HYPOTHERMIA PROTECTION
Numerous studies from a variety of laboratories have investigated the underlying mechanisms by which small reductions in core or central nervous system (CNS) temperature can improve outcomes in models of brain and SCI [7, 8, 34, 37, 38, 71–74]. These mechanisms have been recently summarized in a review article by Dietrich et al. [7]. A major point of discussion is that mild variations in temperature affect many of the pathological mechanisms thought to be important in irreversible neuronal death [4]. Thus, while profound hypothermia was originally thought to primarily work by decreasing O2 consumption and CO2 production, the effects of mild cooling on a variety of other injury consequences besides metabolism have been emphasized [7].
As briefly mentioned, hypothermia can reduce cerebral metabolism and therefore provide energy required for maintaining ionic gradients and other mechanisms important in the normal regulation of cell function [7]. Hypothermia lowers metabolic and energy demands and has been shown to have beneficial effects in regards to adenosine-5’triphosphate (ATP) depletion following cerebral ischemia. In addition to metabolic changes, hypothermic therapy may have significant effects on cerebral blood flow alterations following both brain and SCI [8, 30, 64]. In the early 1950s, for example, studies using systemic hypothermia showed that more profound cooling (25°C) lowered levels of cerebral blood flow. In contrast, other studies have reported with selective brain cooling that cortical blood flow is actually increased with moderate cooling. In the area of SCI, local cooling of spinal cord down to 16°C was shown to decrease blood flow by Hansebout et al. [75]. Although Zielonka et al. [76] reported increases in blood flow when the spinal cord segment was cooled, reported differences may depend on levels of hypothermia, techniques by which systemic or local cooling is introduced, as well as methods of assessing hemodynamic changes.
One of the first pathomechanisms to be evaluated, based on the findings of small variations in temperature, critically affecting neuronal vulnerability was excitotoxicity. Using extracellular microdialysis approaches, Globus et al. [77] demonstrated that mild reductions in brain temperature significantly decreased levels of glutamate and other transmitters released after traumatic brain injury (TBI). These studies that were subsequently replicated in several laboratories showed that a major mechanism by which temperature affected neuronal vulnerability was through excitotoxicity [7]. Similar results were shown following SCI injury in which post-traumatic cooling was reported again to blunt the release of neurotransmitters, including glutamate into the extracellular space [34, 72]. In addition to neurotransmitters, various receptor groups have also been shown to be sensitive to temperature modifications. For example, glutamanergic receptors 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-Methyl-D-asparate acid (NMDA) expression of patterns have been shown to be affected by temperature modifications [78].
The brain and spinal cord are normally protected by the blood-brain and spinal cord barriers that inhibit free passage of blood-borne substances into the parenchyma. This is a major characteristic of the microvasculature of the nervous system and is required for normal CNS function. After brain and SCI, the vascular barrier is commonly disturbed with both ischemia and trauma studies showing extravasation of protein tracers across the brain and spinal cord barriers [79, 80]. Hypothermia protects against abnormal vascular permeability following ischemia and trauma, and with that reduced patterns of white blood cell extravasation into the CNS parenchyma are seen [37, 73]. Recent studies have shown that the beneficial effects of hypothermia on attenuating blood-brain barrier permeability may be through various endothelial dependent processes, including the activation of matrix metalloproteases, which are critical extracellular enzymes that have a variety of biological effects [81].
In addition to these various extracellular mechanisms, intracellular signaling cascades including calcium-dependent pathways are also affected with temperature. For example, after traumatic brain injury, activation of calcium-calmodulin-dependent protein kinase II (a key protein kinase that mediates synaptic strength) has been shown to be attenuated by hypothermia [82, 83]. In other studies, various transcriptional factors that participate in normal neuronal functioning have been shown to be temperature sensitive. For example, immediate early gene c-Fos expression is activated by hypothermia, whereas extracellular signal-regulated protein kinase and c-Jun N-terminal kinase are also affected by temperature modifications [84, 85]. The neuronal cytoskeleton, which is highly vulnerable to injury, is also temperature sensitive. The cytoskeletal protein microtubular-associated protein 2 and β-actin disruption appears to be reversed by hypothermia following brain injury [86, 87].
One of the major mechanisms by which postinjury hypothermia may be working is targeting inflammatory processes [71]. Investigations by many laboratories have emphasized the importance of post-injury inflammatory cascades as clinically relevant secondary injury mechanisms. Recently, therapeutic hypothermia has been shown to target many of the inflammatory cascades that are activated after injury, including the production of pro-inflammatory cytokines, including interleukin-1β, interleukin 18, and tumor necrosis factor-α [88, 89]. Other studies have reported that cooling after injury targets adhesion molecules that are normally upregulated after injury on white cells and endothelial cells, and are critical in recruiting inflammatory cells to an area of injury.
Another temperature sensitive and inflammatory response to injury is the release of reactive oxygens by a variety of brain cells [85]. Postinjury hypothermia has been shown to reduce super oxide, nitric oxide and hydroxal radicals, all believed to be important in neuronal vulnerability after brain injury and SCI [90, 91]. Edema, the swelling of the brain and spinal cord after injury due to water accumulation, is also reduced in some paradigms in which hypothermia is initiated [92, 93]. These studies, using both the regional assessment of tissue water, as well as magnetic resonance imaging have found that hypothermia reduces this clinically relevant consequence of injury [94].
Finally, mechanisms of neuronal cell death have now been shown to be multifactorial and involve necrosis, as well as apoptotic mechanisms [95]. In the area of apoptosis, postinjury hypothermia has been shown to reduce patterns of caspase 3 activation, an important initiator of apoptotic cell death [96]. Other studies have reported that hypothermia after injury reduces the translocation of released cytochrome C [97]. Recent evidence has also turned to the use of screening approaches to assess various genes that may also be sensitive to temperature modifications. Gene array studies have shown that many genes that are upregulated or downregulated after injury appear to be sensitive to temperature manipulations [98]. These families of genes are associated with inflammation, apoptosis, and other cell signaling cascades. It is important to note that the ability of postinjury temperature modifications to affect a variety of genetic and biochemical responses underscores the importance of temperature in the many cellular and molecular responses to CNS injury. Indeed, temperature, before and after an injury, seems to be a critical factor that participates in irreversible neuronal damage and subsequent neuronal dysfunction.
CLINICAL STUDIES EVALUATING SYSTEMIC HYPOTHERMIA FOR ACUTE SCI
As previously emphasized, all previously published clinical studies and case reports on inducible hypothermia for traumatic SCI have implemented local hypothermia at the time of a laminectomy. The human clinical trials were facilitated by the surgical practice of laminectomy and durotomy for the treatment of acute SCI during the 1970s [8]. In these studies, the spinal cord was locally cooled by irrigating the exposed spinal cord or dura with ice cold (4-5°C) saline following the laminectomy. Unfortunately, due to the small sample numbers, lack of randomization, and control groups, heterogeneous treatment methodologies, and other confounding treatments, such as steroids or surgical decompression, these studies lacked the statistical power to justify its widespread use.
Recently, our SCI clinical group performed a retrospective analysis during a 2-year period of a subset of patients with acute complete cervical SCI using a modest intravascular hypothermia protocol [43]. After undergoing a baseline neurological examination without the effects of sedatives, muscle relaxants, alcohol/drug intoxication, or head injury (Glasgow Coma Score (GCS) ≤ 14), patients who presented with a cervical SCI were classified according to the American Spinal Injury Association (AIS). No patients received steroids, such as methylprednisolone, as part of their treatment protocol. An intravascular cooling catheter (CoolGard Icy catheter; Alsius, Irvine, CA) was inserted through the femoral vein using a sterile technique (FIG. 3). The patients were cooled to target temperature (33°C) at the maximum rate (0.5°C/h). Our goal was to maintain our target temperature (33°C) for 48 h. The re-warming phase occurred at a rate of 1°C per 8 h and thus took on average 24- to 32-h to achieve a core temperature of 37°C. After reaching 37°C, the intravenous catheter was removed and the systemic temperature was maintained at 37°C using surface cooling techniques.
Diagram demonstrating the location of the balloon catheter within the inferior vena cava after percutaneous insertion within the femoral vein. (Reprinted with permission from Levi et al. [43]).
Specific exclusion criteria were age greater than 65 years, hyperthermia on admission (T > 38.5°C), severe multi-system injury, active bleeding, pregnancy, coagulopathy, thrombocytopenia, known prior cardiac history, blood dyscrasia, pancreatitis, Reynaud’s syndrome, penetrating spinal column injury (gunshot and knife wounds, and so forth). Patients who were intubated and sedated prior to initial examination by the neurosurgical team, and patients showing an improvement in the neurological exam within 6 h from the injury were also excluded. Our data safety monitoring board specifically looked for potential complications related to the hypothermia including infection, acute respiratory distress syndrome, pneumonia, line sepsis, cardiac abnormalities including arrhythmias, electrolyte abnormalities, deep venous thrombosis, pulmonary embolism, and thrombocytopenia.
There were a total of 14 patients with acute cervical SCI who were entered in to the study. All patients received cervical surgery for either decompression and/or stabilization as part of their treatment. The average initiation time to catheter insertion in this phase I study was 7.40 + 0.27 h (mean ± standard error of the mean). After the intravascular catheter was inserted, the target temperature was achieved within 3 h (2.72 ± 0.42 h) of cooling. The duration of cooling at the target temperature was 47.6 ± 3.1 h. The average total time of cooling was approximately 93.6 ± 4 h. All of the patients underwent surgical intervention and most frequently this was done during the cooling phase or at our target temperature (FIG. 4). There were no apparent adverse effects of temperature related to coagulation or intraoperative hemostasis and/or the development of postoperative hematomas [43].
Temporal changes in temperature in a representative subject. In this patient (patient 9), three distinct phases can be observed: 1) cooling phase, in which the target temperature (33°C) was achieved at an approximate rate of 0.58°C/h; 2) hypothermia phase that lasted for 48 h; and 3) re-warming phase, which allowed for re-establishment of normal temperature (37°C) at 0.1°C = h rate. (Reprinted with permission from Levi et al. [43]).
The intravascular cooling catheters were able to tightly regulate the systemic temperature, as measured by a rectal thermometer. One of the most interesting findings in this study was the significant relationship between body temperature and heart rate, in which the lower temperatures were associated with a lower heart rate. The bradycardia was only treated in cases in which it was believed to be symptomatic, and this was usually manifest by an associated hypotension. The correlation coefficient comparing heart rate and temperature was greater than 0.4 in 10 of 14 patients.
All patients were determined to be an AIS A on admission. Ultimately, 6 patients converted from AIS A to another grade, including 3 patients who converted to AIS B, 2 to AIS C, and 1 to AIS D (FIG. 5) [44]. No patient appeared to worsen as a result of the hypothermia, such as ascending a neurological level [99]. Complications (FIG. 6) were monitored by our data safety monitoring board. The most common complications in the group that received hypothermia were respiratory and included atelectasis (n = 12), pneumonia (n = 8), pleural effusions (n = 8), iatrogenic or traumatic pneumothorax (n = 4), and pulmonary edema (n = 4), followed by adult respiratory distress syndrome (n = 2). One of the patients that had acute respiratory distress syndrome also had aspiration of salt water due to near drowning. Three patients developed arrhythmias, including 1 patient with atrial fibrillation and a controlled ventricular response, 1 with a sinus arrhythmia, and another 1 with an ectopic atrial rhythm. One of them received a transcutaneous pacer. One patient had thrombocytopenia 5 days after hypothermia initiation without clinical manifestation. Anemia was also a frequent finding (n = 11) and appeared to be related to blood loss due to the initial trauma in most patients. Complications, such as acid-base, water, and electrolyte disturbances were very common (n = 7) and were promptly corrected. A high number of urinary tract infections (n = 8) was also observed. All patients received continuous urinary catheterization during their hospital stay, and the Foley catheters were changed every 3 days in an attempt to decrease bacterial contamination. No patient developed a deep venous thrombosis, pulmonary embolism, myocardial infarction, or coagulopathy. No patient died as a direct result of the hypothermia. One patient (62 years old, with a C5 injury) died 1 year after injury of pneumonia. One of the most important findings was the absence of life-threatening complications, such as deep venous thrombosis, pulmonary embolism, and myocardial infarction.
American Spinal Injury Association and International Medical Society of Paraplegia Impairment Scale (AIS) outcome of 14 patients treated with modest hypothermia. After 50.2 [9.7; standard error or the mean (SEM)] weeks, 57.1% of the patients were still AIS A, 21.4% were B, 14.3% were C, and 7.1% were D. In the control group, 11 patients remained AIS A, 1 converted to B, 1 converted to C, and 1 converted to D. A greater number of patients converted to AIS B and C in the hypothermia group (5 patients) when compared with the control (2 patients). There was no statistically significant difference between the final AIS grade in the control and hypothermia groups (2-way analysis of variance). (Reprinted with permission from Levi et al. [44]).
Complications during hospital stay in 14 patients who underwent hypothermia and in 14 control subjects. Respiratory and infectious complications occurred in similar frequency in patients with or without hypothermia. ARDS = adult respiratory distress syndrome; DVT = deep venous thrombosis; MI = myocardial infarction; PE = pulmonary embolism; UGI = upper gastrointestinal bleeding; UTI = urinary tract infection. (Reprinted with permission from Levi et al. [44]).
Moderate systemic hypothermia can be achieved more rapidly and precisely using intravascular techniques. It has been recognized that intravascular infusion with cold saline can lower body temperature quickly [100, 101]. A rapid infusion of iced intravenous fluids (30–40 ml/kg) can reduce core temperatures by 1.6 to 2.5°C in less than 30 minutes; although these fluids are rapid and easy to administer, including the out-of-hospital setting, continuous infusion of large volumes of fluid to maintain a hypothermic core body temperature, this may ultimately lead to cardiovascular instability, particularly in the elderly patient. The newer intravascular cooling catheters rely on a closed circuit of heat exchange permitting iced saline within balloons located in the inferior cava to cool circulating venous blood by means of convection. Using a closed feedback loop, the rate of iced saline infusion into the balloons can be precisely controlled to achieve the required systemic temperature, without the addition of additional fluids. Several commercially available units are manufactured and include the CoolGard Icy catheter used in this study as well as the Celsius control system (Innercool Therapies, San Diego, CA).
One of the areas in which we would like to see a major improvement is the time between SCI and the initiation of hypothermia. In certain cases, the time to admission to hospital was lengthy and the only way to counteract these delays would be to administer cooling “in the field” via intravenous iced saline versus cooling blankets. Another source of delay prior to catheter insertion while in the hospital is the time required to “clear” other potential injuries, such as chest or abdominal injuries. Necessary diagnostic studies, as well as the time taken to image the injured spine and apply traction, also contributed to delays in initiating therapy. With the current catheters, which are magnetic resonance imaging compatible, cooling can be initiated in the trauma bay prior to confirmatory imaging based solely on the clinical diagnosis of SCI.
The application of the intravascular cooling catheters to achieve a core target temperature, including the cooling and re-warming phases, after SCI are emphasized in the current investigations. Challenges in implementing rapid cooling after injury, minimal temperature variation using an intravascular catheter, temperature effect on heart rate, complications, and neurological outcomes are also reported. The current intravascular catheters are powerful vehicles to administer and maintain systemic hypothermia. Recent clinical studies have established the safety of the current devices in the setting of SCI. Given the small sample size and lack of a randomized control group, it was impossible to make any definitive determination regarding potential neuroprotective benefits. In the future, a larger, prospective, randomized, multi-center trial will be required to determine the potential benefits in regard to neuroprotection and recovery from SCIs [102].
CONCLUSIONS
During the last several decades, a significant amount of experimental and clinical work has established therapeutic hypothermia as one of the few effective treatment strategies targeting the brain and SCIs. Mechanisms by which hypothermia may be protective have been discussed and clarified. The ability of mild cooling to target multiple injury cascades emphasizes the multifactorial nature of CNS injury and indicates its use in multiple clinical conditions. Nevertheless, more work needs to be conducted to determine how best to use hypothermia protocols and temperature management strategies to decide which patient populations will benefit most from these treatment procedures.
To combat periods of hyperthermia that may worsen outcome in some patients, temperature management strategies must also be used in situations in which hypothermia may not be safe or effective. Currently, randomized multicenter trials are required to test therapeutic hypothermia in a large number of patients with acute severe SCI [102]. Hopefully, these types of studies will receive the necessary attention and funding that will be required. Only through such a process will the widespread use of hypothermia be accepted in the clinical field of SCI.
References
National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. Birmingham, Alabama: National Spinal Cord Injury Statistical Center, University of Alabama, 2010.
Anderson D, Hall E. Pathophysiology of spinal cord trauma. Ann Emerg Med 1993;22:987–992.
Geisler FH, Coleman WP, Grieco G, et al. The Sygen multicenter acute spinal cord injury study. Spine 2001;26:S87–S98.
Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol 1995;5:407–413.
Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 1990;322:1405–1411.
Cortez R, Levi AD. Acute spinal cord injury. Curr Treat Options Neurol 2007;9:115–125.
Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma 2009;26:301–312.
Guest JD, Dietrich WD. Spinal cord ischemia and trauma. In: Tisherman SA, Sterz F, eds. Therapeutic hypothermia. New York: Springer, 2005: 101–118.
Tator CH, Deecke L. Value of normothermic perfusion, hypothermic perfusion, and durotomy in the treatment of experimental acute spinal cord trauma. J Neurosurg 1973;39:52–64.
Polderman KH. Induced hypothermia and fever control for the prevention and treatment of neurological injuries. Lancet 2008;371:1955–1969.
Albin MS, White RJ, Acosta-Rua G, Yashon D. Study of functional recovery produced by delayed localized cooling of spinal cord injury in primates. J Neurosurg 1968;29:113–120.
Albin M, White R, Locke G. Treatment of spinal cord trauma by selective hypothermic perfusion. Surg Forum 1965;16:423–424.
Albin MS, White RJ, Yashon D, Harris LS. Effects of localized cooling on spinal cord trauma. J Trauma 1969;9:1000–1008.
Downey JA, Chiodi HP, Darling RC. Central temperature regulation in the spinal man. J Appl Physiol 1967;22:91–94.
Koons DD, Gildenberg PL, Dohn DF, Henoch M. Local hypothermia in the treatment of spinal cord injuries. Report of seven cases. Clev Clin Q 1972;39:109–117.
Mecham WF, McPherson WF. Local hypothermia in the treatment of acute injuries of the spinal cord. South Med J 1973;66:95–97.
Negrin J Jr. Spinal cord hypothermia in the neurosurgical management of the acute and chronic post-traumatic paraplegic patient. Paraplegia 1973;10:336–343.
Selker RG. Icewater irrigation of the spinal cord. Surg Forum 1971;22:411–413.
Wells JD, Hansebout RR. Local hypothermia in experimental spinal cord trauma. Surg Neurol 1978;10:200–204.
Dietrich WD. Therapeutic hypothermia for spinal cord injury. Crit Care Med 2009;27:S238-S242.
Kwon BK, Mann C, Sohn HM, et al. Hypothermia for spinal cord injury. Spine 2008;8:859–874.
Lo TP, Cho K-S, Garg MS, et al. Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. J Comp Neurol 2009;514:433–448.
Marion D, Bullock MR. Current and future role of therapeutic hypothermia. J Neurotrauma 2009;26:455–467.
Martinez-Arizala A, Green BA. Hypothermia in spinal cord injury. J Neurotrauma 1992;9(suppl 2):S497–S505.
Yu CG, Jimenez O, Marcillo AE, et al. Beneficial effects of modest systemic hypothermia on locomotor function and histopathological damage following contusion-induced spinal cord injury in rats. J Neurosurg 2000;93:85–93.
Batchelor, PE, Kerr NF, Gatt AM, et al. Hypothermia prior to decompression: buying time for treatment of acute spinal cord injury. J Neurotrauma 2010;27:1357–1368.
Casas CE, Herrera LP, Prusmack C, et al. Effects of epidural hypothermic saline infusion on locomotor outcome and tissue prevention after moderate thoracic spinal cord contusion in rats. Spine 2005;2:308–318.
Hansebout RR, Tanner JA, Romero-Sierra C. Current status of spinal cord cooling in the treatment of acute spinal cord injury. Spine 1984;9:508–511.
Kakinohana M, Taira Y, Marsala M. The effect of graded postischemic spinal cord hypothermia on neurological outcome and histopathology after transient spinal ischemia in rat. Anesthesiology 1999;90:789–798.
Marsala M, Vanicky I, Yaksh TL. Effect of graded hypothermia (27°C to 34°C) on behavioral function, histopathology, and spinal blood flow after spinal ischemia in rat. Stroke 1994;25:2038–2046.
Robertson CS, Foltz R, Grossman RG, et al. Protection against experimental ischemic spinal cord injury. J Neurosurg 1986;64:633–642.
Stauch JT, Lauten A, Spielvogel D, et al. Mild hypothermia protects the spinal cord from ischemic injury in a chronic porcine model. Eur J Cardiothorac 2004;25:708–715.
Tabayashi K, Niibori K, Konno H, et al. Protection from postischemic spinal cord injury by perfusion cooling of the epidural space. Ann Thorac Surg 1993;56:494–498.
Wakamatsu H, Matsumoto M, Nakakimura K, et al. The effects of moderate hypothermia and intrathecal tetracaine on glutamate concentrations of intrathecal dialysate and neurologic and histopathologic outcome in transient spinal cord ischemia in rabbits. Anesth Analg 1999;88:56–62.
Westergren H, Holtz A, Farooque M, et al. Systemic hypothermia after spinal cord compression injury in the rat: does recorded temperature in accessible organs reflect the intramedullary temperature in the spinal cord? J Neurotrauma 1998;15:943–954.
Westergren H, Yu WR, Farooque M, et al. Systemic hypothermia following spinal cord compression injury in the rat: axonal changes studied by beta-APP, ubiquitin, and PGP 9.5 immunohistochemistry. Spinal Cord 1999;37:696–704.
Yu WR, Westergren H, Farooque M, et al. Systemic hypothermia following compression injury of the rat spinal cord: reduction of plasma protein extravasation demonstrated by immunohistochemistry. Acta Neuropathol 1999;98:15–21.
Dietrich WD. Hypothermia treatment potentiates DRK1/2 activation after traumatic brain injury. Eur J Neurosci 2007;26:810–819.
Schmidt KD, Chan CW. Thermoregulation and fever in normal persons and in those with spinal cord injuries. Mayo Clin Proc 1992;67:469–475.
Yu CG, Jagid J, Ruenes G, et al. Detrimental effects of systemic hyperthermia on locomotor function and histopathological outcome after traumatic spinal cord injury in the rat. Neurosurgery 2001;49:152–159.
Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine 2010;35:E57-E62.
Resnick D, Kaiser M, Fehlings M, McCormick P. Hypothermia and human spinal cord injury: position statement and evidence based recommendations. AANS/CNS Joint Section on Disorders of the Spine and the AANS/CNS Joint Section on Trauma. Available at: http://www.spinesection.org/hypothermia.php. Accessed February 10, 2009.
Levi AD, Casella G, Green B, et al. Spinal cord injury and modest hypothermia. J Neurotrauma 2009;26:407–415.
Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery 2010;66:670–677.
Bricolo A, Ore GD, Da Pian R, et al. Local cooling in spinal cord injury. Surg Neurol 1976;6:101–106.
Demian YK, White RJ, Yashon D, et al. Anaesthesia for laminectory and localized cord cooling in acute cervical spine injury. Report of three cases. Br J Anaesth 1971;43:973–979.
Kelly DL Jr, Lassiter KR, Calogero JA, et al. Effects of local hypothermia and tissue oxygen studies in experimental paraplegia. J Neurosurg 1970;33:554–563.
Dimar JR, Shields CB, Zhang YP, et al. The role of directly applied hypothermia in spinal cord injury. Spine 2000;25:2294–2302.
Ha KY, Kim YH. Neuroprotective effect of moderate epidural hypothermia after spinal cord injury in rats. Spine 2008;33:2059–2065.
Hansebout RR, Kuchner EF. Effects of local hypothermia and of steroids upon recovery from experimental spinal cord compression injury. Surg Neurol 1975;4:531–536.
Kuchner EF, Hansebout RR. Combined steroid and hypothermia treatment of experimental spinal cord injury. Surg Neurol 1976;6:371–376.
Downey JA, Miller JM, Darling RC. Thermoregulatory responses to deep and superficial cooling in spinal man. J. Appl Physiol 1969;27;209–212.
Kranke P, Eberhart LH, Roewer N, et al. Pharmacological treatment of postoperative shivering: a quantitative systemic review of randomized controlled trials. Anesth Analg 2002;94:453–460.
Berguer R, Porto J, Fedoronko B, et al. Selective deep hypothermia of the spinal cord prevents paraplegia after aortic cross-clamping in the dog model. J Vasc Surg 1992;15:62–71.
Black JH, Davison JK, Cambria RP. Regional hypothermia with epidural cooling for prevention of spinal cord ischemic complications after thoracoabdominal aortic surgery. Semin Thorac Cardiovasc Surg 2003;15:345–352.
Cambria RP and Davison JK. Regional hypothermia for prevention of spinal cord ischemic complications after thoracoabdominal aortic surgery: experience with epidural cooling. Semin Thorac Cardiovasc Surg 1998;10:61–65.
Cambria RP, Davison JK. Regional hypothermia with epidural cooling for prevention of spinal cord ischemic complications after thoracoabdominal aortic surgery. Semin Thorac Cardiovasc Surg 2000;13:315–324.
Colon R, Frazier OH, Cooley DA, et al. Hypothermic regional perfusion for protection of the spinal cord during periods of ischemia. Ann Thorac Surg 1987;43:639–643.
Fehrenbacher JW, Hart DW, Huddleston E, et al. Optimal end-organ protection for thoracic and thoracoabdominal aortic aneurysm repair using deep hypothermia circulatory arrest. Ann Thorac Surg 2007;83:1041–1046.
Hoshitake A, Mori A, Shimizu H, et al. Use of an epidural cooling catheter with a closed countercurrent lumen to protect against ischemic spinal cord injury in pigs. J Thorac Cardiovasc Surg 2007;134:1220–1226.
Kumar M, Murray MJ, Werner E, et al. Monitoring intrathecal temperature: does core temperature reflect intrathecal temperature during aortic surgery? J Cardiothorac Vasc Anesth 1994;8:35–39.
Malatova Z, Vanicky I, Galik J, et al. Epidural perfusion cooling protects against spinal cord ischemia in rabbits. An evaluation of cholinergic function. Mol Chem Neuropathol 1995;25:81–96.
Svensson LG, Khitin L, Nadolny EM, et al. Systemic temperature and paralysis after thoracoabdominal and descending aortic operations. Arch Surg 2003;138:175–179.
Westergren H, Farooque M, Olsson Y, et al. Spinal cord blood flow charges following systemic hypothermia and spinal cord compression injury: an experimental study in the rat using laser-Doppler flowmetry. Spinal Cord 2001;39:74–84.
Marsala M, Vanicky I, Galik J, Radonak J, Kundrat I, Marsala J. Panmyelic epidural cooling protects against ischemic spinal cord damage. J Surg Res 1993;55:21–31.
Naslund TC, Hollier LH, Money SR, et al. Protecting the ischemic spinal cord during aortic clamping. The influence of anesthetics and hypothermia. Ann Surg 1992;215:409–415.
Botel U, Glaser E, Niedeggen A. The surgical treatment of acute spinal paralysed patients. Spinal Cord 1997;35:420–428.
Green BA, Khan T, Raimondi AJ. Local hypothermia as treatment of experimentally induced spinal cord contusion: quantitative analysis of beneficent effect. Surg Forum 1973;24:436–438.
Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device vs. transection. Exp Neuol 1996;2:244–256.
Pearse DD, Lo TP Jr, Cho KS, et al. Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. J Neurotrauma 2005;22:680–702.
Chatzipanteli K, Yanagawa Y, Marcillo AE, et al. Posttraumatic hypothermia reduces polymorphonuclear leukocyte accumulation following spinal cord injury in rats. J Neurotrauma 2000;17:321–332.
Ishikawa T, Marsala M. Hypothermia prevents biphasic glutamate release and corresponding neuronal degeneration after transient spinal cord ischemia in the rat. Cell Mol Neurobiol 1999;19:199–208.
Lotocki G, Vaccari JP, Perez ER, et al. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of posttraumatic hypothermia. J Neurotrauma 2009;26:1123–1134.
Morino T, Ogata T, Takeba J, Yamamoto H. Microglia inhibition is a target of mild hypothermic treatment after the spinal cord injury. Spinal Cord 2008,46:425–431.
Hansebout RR, Lamont RN, Kamath MV. The effects of local cooling on canine spinal cord blood flow. Can J Neurol Sci 1985;12:83–87.
Zielonka JS, Wagner FC Jr, Dohrmann GJ. Alterations in spinal cord blood flow during local hyperthermia. Surg Forum 1974;25:434–436.
Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem 1995;65:1704–1711.
Friedman LK, Ginsberg MD, Belayev L, et al. Intraischemic but not postischemic hypothermia prevents non-selective hippocampal down regulation of AMPA and NMDA receptor gene expression after globalischemia. Brain Res Mol Brain Res 2001;86:34–47.
Dietrich WO, Busto R, Halley M, Valdes I. The importance of brain temperature in alterations of the blood-brain barrier following cerebral ischemia. J Neuropathol Exp Neurol 1990;49:486–497.
Jiang JY, Lyeth BG, Kapasi MZ, Jenkins LW, Povlishock JT. Moderate hypothermia reduces blood-brain barrier disruption following traumatic brain injury in the rat. Acta Neuropathol (Berl) 1992;84:495–500.
Truettner JS, Alonso OF, Dietrich WD. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J Cereb Blood Flow Metab 2005;25:1505–1516.
Hu BR, Kamme F, Wieloch T. Alterations of Ca2+/calmodulin-dependent protein kinase II and its messenger RNA in the rat hippocampus following normo- and hypothermic ischemia. Neuroscience 1995;68:1003–1016.
Shimohata T, Zhao H, Sung JH, Sun G, Mochly-Rosen D, Steinberg GK. Suppression of delta PKC activation after focal central ischemia contributes to the protective effect of hypothermia. J Cereb Blood Flow Metab 2007;27:1463–1475.
Atkins CM, Oliva AA Jr, Alonso OF, et al. Hypothermia treatment potentiates DRK1/2 activation after traumatic brain injury. Eur J Neurosci 2007;26:810–819.
Hicks SD, Parmele KT, De Franco DB, Klann E, Callaway CW. Hypothermia differentially increases extracellular signal-regulated kinase and stress-activated protein kinase/c-Jun terminal kinase activation in the hippocampus during reperfusion after asphyxia cardiac arrest. Neuroscience 2000;98:677–685.
Haranishi Y, Kawata R, Fukuda S, et al. Moderate hypothermia, but not calpain inhibitor 2, attenuates the protolysis of microtubule-associated protein 2 in the hippocampus following traumatic brain injury in rats. Eur J Anaesthesiol 2005;22:140–147.
Taft WC, Yang K, Dixon CE, Clifton GL, Hayes RL. Hypothermia attenuates the loss of hippocampal microtubule-associated protein 2 (MAP2) following traumatic brain injury. J Cereb Blood Flow Metab 1993;13:796–802.
Goss JR, Styren SD, Miller PD, et al. Hypothermia attenuates the normal increase in interleukin 1 beta RNA and nerve growth factor following traumatic brain injury in the rat. J Neurotrauma 1995;12:159–167.
Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonzo OF, Dietrich WD. Interleukin-1 b messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats; importance of injury severity and brain temperature. Neurosurgery 2002;51:195–203.
Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari, MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci 2002;22:3921–3928.
Maier CM, Sun GH, Cheng D, Yenari MA, Chan PH, Steinberg GK. Effects of mild hypothermia on superoxide anion production superoxide dismutase expression, and activity following transient focal cerebral ischemia. Neurobiol Dis 2002;11:28–42.
Kawai N, Nakamura T, Nagao S. Effects of brain hypothermia on brain edema formation after intracerebral hemorrhage in rats. Acta Neurochir Suppl 2002;81:233–235.
Park CK, Jun SS, Kim MC, Kang JK. Effects of systemic hypothermia and selective brain cooling on ischemic brain damage and swelling. Acta Neurochir Suppl 1998;71:225–228.
Mancuso A, Derugin N, Hara K, Sharp FR, Weinstein PR. Mild hypothermia decreases the incidence of transient ADC reduction detected with diffusion MRI and expression of c-fos and hsp70 mRNA during acute focal ischemia in rats. Brain Res 2000;887:34–45.
Keane RW, Kraydieh S, Lotocki G, et al. Apoptotic and anti-apoptotic mechanisms following spinal cord injury. J Neuropathol Exp Neurol 2001;60:422–429.
Lotocki G, de Rivero Vaccari JP, Perez ER, et al. Therapeutic hypothermia modulates TNFR1 signaling in the traumatized brain via early transient activation of the JNK pathway and suppression of XIAP cleavage. Eur J Neurosci 2006;24:2283–2290.
Yenari MA, Iwayama S, Cheng D, et al. Mild hypothermia attenuates cytochrome c release but does not alter BcI-2 expression or caspase activation after experimental stroke. J Cereb Blood Flow Metab 2002;22:29–38.
Kobayashi MS, Asai S, Ishikawa K, Nishida Y, Nagata T, Takahashi Y. Global profiling of influence of Intraischemic brain temperature on gene expression in rat brain. Brain Res Rev 2008;58:171–191.
Belanger E, Picard C, Lacerte D, Lavallee P, Levi AD. Subacute posttraumatic ascending myelopathy after spinal cord injury: report of three cases. J Neurosurg 2000;93:294–299.
Rajek A, Greif R, Sessler DI, et al. Core cooling by central venous infusion of ice-cold (4°C and 20°C) fluid: isolation of core and peripheral thermal compartments. Anesthesiology 2000;93:629–637.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–563.
Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007;45:190–205.
Acknowledgments
The authors thank Jeremy Lytle for editorial assistance. Full conflict of interest disclosure is available in the electronic supplementary material for this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 266 kb)
Rights and permissions
About this article
Cite this article
Dietrich, W.D., Levi, A.D., Wang, M. et al. Hypothermic Treatment for Acute Spinal Cord Injury. Neurotherapeutics 8, 229–239 (2011). https://doi.org/10.1007/s13311-011-0035-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-011-0035-3