Abstract
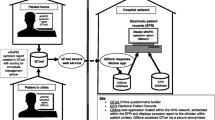
The National Cancer Institute (NCI) is developing a patient-reported version of its Common Terminology Criteria for Adverse Events, called the “PRO-CTCAE.” The PRO-CTCAE consists of a library of patient-reported items which can be administered in clinical trials to directly capture the patient experience of adverse events during cancer treatment, as well as a software platform for administering these items via computer or telephone. In order to better understand the impressions of stakeholders involved in cancer clinical research about the potential value of the PRO-CTCAE approach to capturing adverse event information in clinical research, as well as their perspectives about barriers and strategies for implementing the PRO-CTCAE in NCI-sponsored cancer trials, a survey was conducted. A survey including structured and open-ended questions was developed to elicit perceptions about the use of patient-reported outcomes (PROs) for adverse event reporting, and to explore logistical considerations for implementing the PRO-CTCAE in cancer trials. The survey was distributed electronically and by paper to a convenience sample of leadership and committee members in the NCI’s cooperative group network, including principal investigators, clinical investigators, research nurses, data managers, patient advocates, and representatives of the NCI and Food and Drug Administration. Between October, 2008 through February, 2009, 727 surveys were collected. Most respondents (93%) agreed that patient reporting of adverse symptoms would be useful for improving understanding of the patient experience with treatment in cancer trials, and 88%, 80%, and 76%, respectively, endorsed that administration of PRO-CTCAE items in clinical trials would improve the completeness, accuracy, and efficiency of symptom data collection. More than three fourths believed that patient reports would be useful for informing treatment dose modifications and towards FDA regulatory evaluation of drugs. Eighty-eight percent felt that patients in clinical trials would be willing to self-report adverse symptoms at clinic visits via computer, and 68% felt patients would self-report weekly from home via the internet or an automated telephone system. Lack of computers and limited space and personnel were seen as potential barriers to in-clinic self-reporting, but these were judged to be surmountable with adequate funding. The PRO-CTCAE items and software are viewed by a majority of survey respondents as a means to improve adverse event data quality and comprehensiveness, enhance clinical decision-making, and foster patient-clinician communication. Research is ongoing to assess the measurement properties and feasibility of implementing this measure in cancer clinical trials.
Similar content being viewed by others
References
National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. NIH publication # 09-7473. Published May 29, 2009; Revised Version 4.02 September 15, 2009 (available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf, last accessed December 30, 2010).
Trotti, A., Colevas, A. D., et al. (2003). CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in Radiation Oncology, 13(3), 176–181.
Trotti, A., Colevas, A. D., Setser, A., & Basch, E. (2007). Patient-reported outcomes and the evolution of adverse event reporting in oncology. Journal of Clinical Oncology, 25(32), 5121–5127.
Fromme, E. K., Eilers, K. M., et al. (2004). How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. Journal of Clinical Oncology, 22(17), 3485–3490.
Basch, E. (2010). The missing voice of patients in drug-safety reporting. The New England Journal of Medicine, 362(10), 865–869.
Pakhomov, S. V., Jacobsen, S. J., Chute, C. G., & Roger, V. L. (2008). Agreement between patient-reported symptoms and their documentation in the medical record. American Journal of Managed Care, 14, 530–539.
Schnadig, I. D., Fromme, E. K., et al. (2008). Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer, 113(8), 2205–2214.
Parliament, M. B., Danjoux, C. E., et al. (1985). Is cancer treatment toxicity accurately reported? International Journal of Radiation Oncology, Biology, Physics, 11(3), 603–608.
Basch, E., Iasonos, A., McDonough, T., Barz, A., Culkin, A., Kris, M. G., et al. (2006). Clinician versus patient self-reporting of symptoms during cancer treatment: a paired analysis using the national cancer institute’s common terminology criteria for adverse events (CTCAE). The Lancet Oncology, 7(10), 903–909.
Bruner, D. W., Scott, C., et al. (1995). RTOG’s first quality of life study–RTOG 90-20: a phase II trial of external beam radiation with etanidazole for locally advanced prostate cancer. International Journal of Radiation Oncology Biology and Physics, 33(4), 901–906.
Varricchio, C. G., & Sloan, J. A. (2002). The need for and characteristics of randomized, phase III trials to evaluate symptom management in patients with cancer. Journal of the National Cancer Institute, 94(16), 1184–1185.
U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for industry: Patient-reported outcomes measures: Use in medical product development to support labeling claims. Issued December 2009 (available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf, last accessed February 9, 2011).
Willke, R. J., Burke, L. B., et al. (2004). Measuring treatment impact: a review of patient-reported outcomes and other efficacy endpoints in approved product labels. Controlled Clinical Trials, 25(6), 535–552.
Bruner, D. W., Bryan, C. J., Aaronson, N., Blackmore, C. C., Brundage, M., Cella, D., et al. (2007). Issues and challenges with integrating patient-reported outcomes in clinical trials supported by the national cancer institute-sponsored clinical trials network. Journal of Clinical Oncology, 25(32), 5051–5057.
Velikova, G. L., Booth, et al. (2004). Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. Journal of Clinical Oncology, 22(4), 714–724.
Sloan, J. A., Cella, D., et al. (2005). Clinical significance of patient-reported questionnaire data: another step toward consensus. Journal of Clinical Epidemiology, 58(12), 1217–1219.
Lipscomb, J., Reeve, B. B., et al. (2007). Patient-reported outcomes assessment in cancer trials: taking stock, moving forward. Journal of Clinical Oncology, 25(32), 5133–5140.
Patrick, D. L., Ferketich, S. L., et al. (2003). National institutes of health state-of-the-science conference statement: symptom management in cancer: pain, depression, and fatigue, July 15–17, 2002. Journal of the National Cancer Institute, 95(15), 1110–1117.
Rock, E. P., Kennedy, D. L., et al. (2007). Patient-reported outcomes supporting anticancer product approvals. Journal of Clinical Oncology, 25(32), 5094–5099.
Basch, E., Artz, D., Dulko, D., Scher, K., Sabbatini, P., Hensley, M., et al. (2005). Patient online self-reporting of toxicity symptoms during chemotherapy. Journal of Clinical Oncology, 23(15), 3552–3561.
Abernethy, A. P., Ahmad, A., Zafar, S. Y., Wheeler, J. L., Reese, J. B., & Lyerly, H. K. (2010). Electronic patient-reported data capture as a foundation of rapid learning cancer care. Medical Care, 48(6 Suppl), S32–S38.
Committee on Cancer Clinical Trials and the NCI Cooperative Group Program, Institute of Medicine. (2010). A national cancer clinical trials system for the 21st century: reinvigorating the NCI Cooperative group program. Washington: Institute of Medicine of the National Academies.
Institute of Medicine, National Academy of Sciences. (2010). A national cancer clinical trials system for the 21st century: reinvigorating the NCI cooperative group program. Washington, DC: National Academies Press.
Buetow, K. H. (2009). An infrastructure for interconnecting research institutions. Drug Discovery Today, 14(11–12), 605–610.
Chilukuri, R., Gulati, M., Agarwal, H., Baumgartner, P., Reeve, B., Sit, L., et al. (2009). Software system for administering the Patient-Reported Outcomes version of the CTCAE (PRO-CTCAE). Presented at the National Cancer Institute caBIG Annual Meeting, Washington, DC.
Basch, E., Reeve, B. B., Cleeland, C. S., Sloan, J. A., Mendoza, T. R., Abernethy, A. P., et al. (2010). Development of the patient-reported version of the common terminology criteria for adverse events (PRO-CTCAE). Presented at 2010 ASCO Annual Meeting, Chicago, IL. J Clin Oncol 28 (suppl; abstr e19605).
Hay, J., Atkinson, T. M., Mendoza, T. R., Reeve, B. B., Willis, G., Gagne, J. J., et al. (2010). Refinement of the patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) via cognitive interviewing. Presented at 2010 ASCO Annual Meeting, Chicago, IL. J Clin Oncol 28:15s (suppl; abstr 9060).
Dueck, A. C., Mendoza, T. R., Reeve, B. B., Sloan, J. A., Cleeland, C. S., Hay, J., et al. (2010) Validation study of the patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Presented at 2010 ASCO Annual Meeting, Chicago, IL. J Clin Oncol 28:15s (suppl; abstr TPS274).
Acknowledgments
This work was supported by the National Cancer Institute under contract HHSN261200800043C awarded to Dr. Ethan Basch, PI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Implications
Policymakers: Patient self-reporting provides essential information about the safety and effectiveness of drugs and devices which can aid setting treatment and reimbursement priorities.
Researchers: Patient-reported outcomes provide reliable and valid accounts of the symptoms experienced by patients during treatment, which can provide important study data and prompt new research questions.
Practitioners: Systems that allow patients to self-report their own side effects in real-time to providers have been demonstrated to improve symptom control, patient satisfaction, patient-clinician communication, and to aid in clinical decision-making.
APPENDIX
APPENDIX




About this article
Cite this article
Bruner, D.W., Hanisch, L.J., Reeve, B.B. et al. Stakeholder perspectives on implementing the National Cancer Institute’s patient-reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Behav. Med. Pract. Policy Res. 1, 110–122 (2011). https://doi.org/10.1007/s13142-011-0025-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13142-011-0025-3