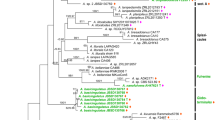
Abstract
Sabellastarte Krøyer, 1856 (Sabellidae), a morphologically homogeneous group distributed in warm and temperate coasts of the Indo-Pacific and Caribbean Sea, is characterized by the presence of a unique combination of features. To date, the genus comprises eight species, but morphological characters traditionally used in diagnostics have shown intra-specific variability, making species boundaries and distributions unclear. The present study constitutes the first attempt to test the monophyly of Sabellastarte and its relationships to other sabellid genera by combining molecular (COI and 16S) and morphological data. Results include placement of a clade containing Stylomma, Sabella, Branchiomma and Bispira as the sister group to Sabellastarte. Phylogenetic analyses and genetic divergence among specimens from several localities around the world indicate the presence of at least six lineages within Sabellastarte. In the context of a discussion of species boundaries and diagnostic features, the distribution of some of those lineages can be explained by the presence of cryptic species and potential introductions.





Similar content being viewed by others
References
Bybee, D. R., Bailey-Brock, J. H., & Tamaru, C. S. (2006a). Larval development of Sabellastarte spectabilis (Grube 1878) (Polychaeta: Sabellidae) in Hawaiian waters. In R. Sardá, G. San Martín, E. López, D. Martín, & D. George (Eds.), Advances in Polychaete Research (pp. 279–286). Barcelona: Scientia Marina 70S3.
Bybee, D. R., Bailey-Brock, J. H., & Tamaru, C. S. (2006b). Evidence for sequential hermaphroditism in Sabellastarte spectabilis (Grube 1878) (Polychaeta: Sabellidae). Pacific Science, 60, 541–547.
Bybee, D. R., Bailey-Brock, J. H., & Tamaru, C. S. (2007). Gametogenesis and spawning periodicity in the fan worm Sabellastarte spectabilis (Grube 1878) (Polychaeta: Sabellidae). Marine Biology, 151, 639–648.
Bybee, S. M., Ogden, T. H., Branham, M. A., & Whiting, M. F. (2008). Molecules, morphology and fossils: a comprehensive approach to odonate phylogeny and the evolution of the odonate wing. Cladistics, 23, 1–38.
Capa, M. (2007). Taxonomic revision and phylogenetic relationships of apomorphic sabellids (Polychaeta) from Australia. Invertebrate Systematics, 21, 537–567.
Capa, M. (2008). The genera Bispira Krøyer, 1856 and Stylomma Knight-Jones, 1997 (Polychaeta, Sabellidae): systematic revision, relationships with close related taxa and new species from Australia. Hydrobiologia, 596, 301–327.
Capa, M., Hutchings, P., Aguado, M. T., & Bott, N. (In press). Phylogeny of Sabellidae (Annelida) and relationships with related taxa inferred from morphology and multiple genes. Cladistics.
Coles, S. L., DeFelice, R. C., Elredge, L. G., & Carlton, J. T. (1999). Historical and recent introductions of non-indigenous marine species into Perl Harbour, O’ahu, Hawaiian islands. Marine Biology, 135, 1247–158.
Coles, S. L., & Eldredge, L. G. (2002). Nonindigenous species introductions on coral reefs: A need for information. Pacific Science, 56, 191–209.
Costa-Paiva, E. M., Paiva, P. C., & Klautau, M. (2007). Anaesthetization and fixation effects on the morphology of sabellid polychaetes (Annelida: Polychaeta: Sabellidae). Journal of the Marine Biological Association of the United Kingdom, 87, 1127–1132.
Dawson, M. N. (2005). Incipient speciation of Catostylus mosaicus (Scyphozoa, Rhizostomeae, Catostylidae), comparative phylogeography and biogeography in south-east Australia. Australian Journal of Biogeography, 32, 515–533.
Division of Land and Natural Resources, State of Hawaii (1976–2008). Catch Data. Hawaii State Government. http://hawaii.gov/dlnr/.
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–97.
Erséus, C., & Kvist, S. (2007). COI variation in Scandinavian marine species of Tubificoides (Annelida: Clitellata: Tubificidae). Journal of the Marine Biological Association of the United Kingdom, 87, 1121–1126.
Farris, J. S., Albert, V. A., Källersjö, M., Lipscomb, D., & Kluge, A. G. (1996). Parsimony jackknifing outperforms neighbor-joining. Cladistics, 12, 99–124.
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39, 783–791.
Fitzhugh, K. (1989). A systematic revision of the Sabellidae-Caobangiidae-Sabellongidae complex (Annelida: Polychaeta). Bulletin of the American Museum of Natural History, 192, 1–104.
Fitzhugh, K. (2003). A new species of Megalomma Johansson, 1927 (Polychaeta: Sabellidae: Sabellinae) from Taiwan, with comments on sabellid dorsal lip classification. Zoological Studies, 42, 106–134.
Fitzhugh, K., & Rouse, G. W. (1999). A remarkable new genus and species of fan worm (Polychaeta: Sabellidae: Sabellinae) associated with marine gastropods. Invertebrate Biology, 118, 357–390.
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
Giangrande, A., Licciano, M., Pagliara, A., & Gambi, M. C. (2000). Gametogenesis and larval development in Sabella spallanzanii (Polychaeta: Sabellidae) from the Mediterranean Sea. Marine Biology, 136, 847–861.
Giangrande, A., & Petraroli, A. (1994). Observations on reproduction and growth of Sabella spallanzanii (Polychaeta, Sabellidae) in the Mediterranean Sea. Memoires du Muséum National d’Histoire Naturelle de Paris, 162, 51–56.
Goloboff, P. A., Farris, J. S., & Nixon, K. (2003). TNT: Tree analysis using New Technology. Version 1.0. http://www.zmuc.dk/public/phylogeny/tnt/. Accessed 1 November, 2009.
Goloboff, P. A., Farris, J. S., & Nixon, K. (2008). TNT, a free program for phylogenetic analysis. Cladistics, 24, 774–786.
Halt, M. N., Kupriyanova, E. K., Cooper, S. J. B., & Rouse, G. W. (2009). Naming species with no morphological indicators: species status of Galeolaria caespitosa (Annelida: Serpulidae) inferred from nuclear and mitochondrial gene sequences and morphology. Invertebrate Systematics, 23, 205–222.
Hilario, A., Johnson, S. B., Cunha, M. R., & Vrijenhoek, R. C. (2010). High diversity of frenulates (Polychaeta: Siboglinidae) in the Gulf of Cadiz mud volcanoes: a DNA taxonomy analysis. Deep-Sea Reseach I., 57, 143–150.
Knight-Jones, P. (1983). Contributions to the taxonomy of Sabellidae (Polychaeta). Zoological Journal of the Linnaean Society, 79, 245–295.
Knight-Jones, P. (1994). Two new species of Branchiomma (Sabellidae) with descriptions of closely related species and comments on Pseudobranchiomma and Sabellastarte. Memoires du Muséum National d’Histoire Naturelle, 162, 191–198.
Knight-Jones, P., & Bowden, N. (1984). Incubation and scissiparity in Sabellidae (Polychaeta). Journal of the Marine Biological Association of the United Kingdom, 64, 809–818.
Knight-Jones, P., & Giangrande, A. (2003). Two new species of an atypical group of Pseudobranchiomma Jones (Polychaeta: Sabellidae). Hydrobiologia, 496, 95–103.
Knight-Jones, P., & Mackie, A. S. Y. (2003). A revision of Sabellastarte (Polychaeta: Sabellidae). Journal of Natural History, 37, 2269–2301.
Knight-Jones, P., & Perkins, T. H. (1998). A revision of Sabella, Bispira, and Stylomma (Polychaeta: Sabellidae). Zoological Journal of the Linnaean Society, 123, 385–467.
Kupriyanova, E. K., & Rouse, G. W. (2008). Yet another example of paraphyly in Annelida: Molecular evidence that Sabellidae contains Serpulidae. Molecular Phylogenetics and Evolution, 46, 1174–1181.
Licciano, M., & Giangrande, A. (2008). The genus Branchiomma (Polychaeta: Sabellidae) in the Mediterranean Sea, with the description of B. maerli n. sp. Scientia Marina, 72, 383–391.
Nei, M., & Kumar, S. (2000). Molecular evolution and phylogenetics. New York: Oxford University Press.
Nogueira, J. M. M., & Knight-Jones, P. (2002). A new species of Pseudobranchiomma Jones (Polychaeta: Sabellidae) found amongst Brazilian coral, with a redescription of P. punctata (Treadwell, 1906) from Hawaii. Journal of Natural History, 36, 1661–1670.
Nylander, J. A. A., Ronquist, F., Huelsenbeck, J. P., & Nieves-Aldrey, J. L. (2004). Bayesian phylogenetic analysis of combined data. Systematic Biology, 53, 74–67.
O’Hara, T. D., & Poore, G. C. B. (2000). Patterns of distribution for southern Australian marine echinoderms and decapods. Journal of Biogeography, 27, 1321–1335.
Osborn, K. J., Rouse, G. W., Goffredi, S. K., & Robison, B. H. (2007). Description and relationships of Chaetopterus pugaporcinus, an unusual pelagic polychaete (Annelida, Chaetopteridae). Biological Bulletin, 212, 40–54.
Page, R. (1998). Nexus data editor. Available from http://taxonomy.zoology.gla.ac.uk/rod/NDE/nde.html. Accessed 3 September 2010.
Patti, F. P., Gambi, M. C., & Giangrande, A. (2003). A preliminary study on the systematic relationships of Sabellinae (Polychaeta, Sabellidae), based on the C1 domain of the 28 S rDNA, with discussion of reproductive features. Italian Journal of Zoology, 70, 269–278.
Pleijel, F., Rouse, G., & Nygren, A. (2009). Five colour morphs and three new species of Gyptis (Hesionidae, Annelida) under a jetty in Edithburgh, South Australia. Zoologica Scripta, 38, 89–99.
Posada, D., & Crandall, K. A. (1998). MODELTEST: testing the model of DNA substitution. Bioinformatics, 14, 817–818.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Ronquist, F., Huelsenbeck, J. P., & van der Mark, P. (2005). MrBayes 3.1 Manual. http://mrbayes.csit.fsu.edu/mb3.1_manual.pdf. Accessed 1 September 2008.
Rouse, G. W., & Fitzhugh, K. (1994). Broadcasting fables: is external fertilization really primitive? Sex, size, and larvae in sabellid polychaetes. Zoologica Scripta, 23, 271–312.
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H., & Flook, P. (1994). Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America, 87, 651–701.
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22, 2688–2690.
Stamatakis, A., Hoover, P., & Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web server. Systematic Biology, 57, 758–771.
Swofford, D. L. (2002). PAUP*: Phylogenetic analysis using parsimony (*and other methods). Sunderland: Sinauer Associates.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Tovar-Hernández, M. A., & Knight-Jones, P. (2006). Species of Branchiomma (Polychaeta: Sabellidae) from the Caribbean Sea and Pacific coast of Panama. Zootaxa, 1189, 1–37.
Tovar-Hernández, M. A., & Salazar-Vallejo, S. I. (2006). Sabellids (Polychaeta: Sabellidae) from the Grand Caribbean. Zoological Studies, 45, 24–66.
Virgilio, M., Fauvelot, C., Constantini, F., Abbiati, M., & Backeljau, T. (2009). Phylogeography of the Common Ragworm Hediste diversicolor (Polychaeta: Nereididae) reveals cryptic diversity and multiple colonization events across its distribution. Molecular Ecology, 18, 1980–1994.
Vrijenhoek, R. C., Johnson, S. B., & Rouse, G. W. (2009). A remarkable diversity of bone-eating worms (Osedax; Siboglinidae; Annelida). BMC Biology, 7, 74.
Walsh, W. J., Cotton, S. P., Dierking, J., & Williams, I. D. (2003). The commercial marine aquarium fishery in Hawai’i 1976–2003. In A. M. Friedlander (Ed.), Status of Hawai’i’s coastal fisheries in the new millennium. Proceedings of a Symposium sponsored by the American Fisheries Society, Hawai’i chapter (pp. 132–159). Honolulu: Hawaiian Audubon Society.
Waters, M. J. (2008). Marine biogeographical disjunction in temperate Australia: historical landbridge, contemporary currents, or both? Diversity and Distributions, 14, 692–700.
Wirchansky, B. A., & Shain, D. H. (2010). A new species of Haemopis (Annelida: Hirudinea): evolution of North American terrestrial leeches. Molecular Phylogenetics and Evolution, 54, 226–234.
Acknowledgements
We would like to thank numerous colleagues that have made this study possible by helping us collect or sending specimens from various parts of the world. These are Pat Hutchings, Kate Attwood, Anna Murray and Steve Keable (Australian Museum, Australia), Chris Glasby and Belinda Álvarez de Glasby (Museum and Art Galleries of the Northern Territory, Australia), Robin Wilson (Museum Victoria, Australia), Roger Goodwill (Brigham Young University-Hawaii, USA), Ana Rubio (Fisheries, Australia), Javed Mustaquim (University of Pakistan, Pakistan), María Ana Tovar-Hernández (Universidad Nacional Autónoma de México, Mexico), Tetsuya Kato and Mitsuru Ohta (Shirahama Aquarium at Kyoto University, Japan), Eijiroh Nishi (Yokohama National University, Japan), Douglas Fenner (Department of Marine and Wildlife Resources, American Samoa), and Greg Rouse (Scripps Institution of Oceanography, USA). We are also grateful to Brigham Young University Provo and particularly to Michael Whiting and the Whiting Laboratory of Molecular Systematics for the use of their facilities and lab space. Rebecca Johnson (Australian Museum) assisted in the molecular laboratory. We wish to thank the Willi Hennig Society for allowing free access to TNT software. Finally, we would like to thank two anonymous reviewers and the editors, Martin V. Sørensen and Olaf Bininda-Emonds, for useful comments and suggestions which helped improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendices
Appendix 1 Material examined
Mexico
Veracruz: AM W31846 (1 specimen), BYUHMNH E.007.
Samoa
Samoa: AM W.32478 (1 spec.), AM W.32479 (1 spec.).
Philippines
Bohol Islands: MPW 374. Lectotype.
Malaysia
Kota Kinabalu: AM W.30001 (1 spec.), AM W30384 (1 spec.), AM W30386 (1 spec.).
Hawaii
Oahu, Kaneohe: AM W.31843 (1 spec.).
Coconut Island: BYUHMNH E.002 (1 spec.).
Saipan
Northern Marianas Islands: AM W.31847 (1 spec.), BYUHMNH.E.008 (1 spec.).
Pakistan
Karachi, Hawkes Bay: AM W.31823 (1 spec.), AM W.31824 (1 spec.), AM W.31825 (1 spec.).
Japan
Sagami Bay: AM W.81845 (1 spec.), AM W.320202 (1 spec.), BYUHMNH E.003 (1 spec.), BYUHMNH E.004 (1 spec.), BYUHMNH E.005 (1 spec.), BYUHMNH E.006 (1 spec.).
Australia, New South Wales
Port Jackson: AM G.2045 (1 spec.), AM G.11203 (2 spec.), AM G.11204 (1 spec.), AM W.24905 (1 spec.), AM W.20625 (1 spec.), AM W.29659 (1 spec.), AM W.29658 (1 spec.), AM W.32007 (1 spec.), AM W.4280 (1 spec.), AM W.6194 (2 spec.), AM W.4408 (1 spec.), AM W.6538 (1 spec.), AM W.1763 (1 spec.), AM W.24279 (1 spec.).
Solitary Islands: AM W.29657 (1 spec.).
Newcastle: AM W.34243 (1 spec.), AM W.34771 (3 spec.), AM W.17844 (1 spec.).
North Sydney: AM W.6193 (1 spec.), AM W.6195 (1 spec.), AM W.24904 (1 spec.), AM W.5706 (1 spec.).
Port Stephens: AM W.33910 (1 spec.), AM W.30383 (1 spec.), AM W.30383 (1 spec.).
Port Hacking: AM W.24899 (3 spec.).
Botany Bay: AM W.34770 (1 spec.).
Port Kembla: AM W.34244 (1 spec.), AM W.34245 (1 spec.), AM W.34246 (1 spec.), AM W.34772 (1 spec.), AM W.32012 (1 spec.).
Batemans Bay: AM W.31105 (4 spec.).
Eden: AM W.34242 (1 spec.).
Twofold Bay: AM W.199766 (1 spec.), AM W.199769 (3 spec.), AM W.199765 (3 spec.), AM W.199767 (1 spec.), AM W.199768 (1 spec.), AM W.199770 (1 spec.), AM W.199771 (1 spec.), AM W.199772 (1 spec.).
Victoria
Port Phillip Bay: AM W. 11967 (1 spec.), AM W. 14129 (1 spec.).
Northern Territory
Darwin: AM W.29656 (1 spec.), NTM W.18990 (2 spec.), NTM W.53 (1 spec.), NTM W.54 (1 spec.), NTM W.55 (1 spec.), NTM W.57 (1 spec.), NTM W.58 (1 spec.), NTM W.59 (1 spec.), NTM W.17359 (1 spec.), AM W.31844 (1 spec.).
Western Australia
Dampier Archipelago: AM W.30024.
Kimberleys: AM W.31826 (1 spec.), AM W.31827 (1 spec.), AM W.31828 (1 spec.), AM W.31829 (1 spec.), AM W.31830 (1 spec.).
Appendix 2 List of morphological characters and character states
Char. 1–11 Branchial crown and other prostomial appendages
-
1
Crown lobe shape: (0) two semicircles or slightly involuted ventrally, never forming one whorl; (1) both halves involuted ventrally in more than one whorl; (2) only one half spiraled.
-
2
Inter-digitating radioles: (0) absent; (1) present.
-
3
Radiolar eyes: (0) absent; (1) present.
-
4
Type of radiolar eyes: (0) simple eyespots; (1) unpaired distal compound eyes; (2) paired compound eyes; (3) unpaired compound eyes.
-
5
Stylodes: (0) absent; (1) present.
-
6
Number of cells in radiolar ‘skeleton’: (0) four; (1) four to ten; (2) more than ten.
-
7
Dorsal basal flange: (0) absent; (1) present.
-
8
Dorsal radiolar appendages: (0) short, i.e. less than 1/4 of crown; (1) long, i.e. more than 1/4 of crown.
-
9
Skeleton of dorsal radiolar appendages: (0) absent; (1) present.
-
10
Dorsal pinnular appendages: (0) absent; (1) present.
-
11
Ventral sacs: (0) within crown base; (1) outside of crown; (2) absent.
Sabellastarte and some members of Bispira present ventrally involuted or spiraled crown lobes (Fig. 1i), whereas Sabella spallanzanii (Gmelin, 1791) has only one side of the crown that is spiralled (Fig. 1a). The remaining terminal taxa show semicircular or slightly involuted, brown lobes. Some individuals of Sabellastarte have inter-digitating radioles appearing as a second internal row (Knight-Jones and Mackie 2003). Sabella and Sabellastarte typically lack radiolar eyes, similar to some members of Bispira and Pseudobranchiomma, while members of Branchiomma characteristically have several pairs of compound eyes along radioles, and Stylomma has unpaired subterminal eyes or some randomly distributed eyespots (e.g. Capa 2008). Other radiolar structures found in the ingroup are the stylodes, unique to members of Branchiomma, and the radiolar flanges, present in Bispira, Pseudobranchiomma and Stylomma. The number of longitudinal rows of cells forming the ‘radiolar skeleton’ has traditionally been used as a diagnostic generic feature among sabellids (Fitzhugh 1989; Fitzhugh and Rouse 1999; Rouse and Fitzhugh 1994). The character states have been modified from previous studies to accommodate the variability shown in the taxa considered for the present study. Members of Sabellastarte have a dorsal basal flange on the dorsal margin of the crown (Fig. 1g), shared with members of Stylomma (Capa 2008) and with some species of Pseudobranchiomma (Nogueira and Knight-Jones 2002) and Pseudopotamilla (Capa 2007). All ingroup terminals except one show dorsal lips with dorsal radiolar appendages (Fig. 1h, i) supported by a radiolar ‘skeleton’ (Fitzhugh 2003); the exception is Stylomma (authors’ pers. obs.), but the attribute is also absent in Pseudopotamilla. Members of Sabellastarte and Stylomma have ventral sacs located inside the crown lobes (Fig. 1h, j), as in the outgroup, while in the remaining ingroup terminals the sacs are located outside the radiolar crown (Fig. 1b) (Capa 2008; Knight-Jones and Perkins 1998).
Char. 12–15 Peristomium and collar
-
12
Collar dorsal margins: (0) fused to the faecal groove; (1) margins of collar free, dorsal.
-
13
Dorsal notches: (0) absent; (1) present.
-
14
Dorsal pockets: (0) absent; (1) present.
-
15
Ventro-lateral notches: (0) absent; (1) present.
The posterior peristomial ring collar dorsal margins are fused to the faecal groove in Sabellastarte (Fig. 1i, l), Sabella (Fig. 1c), and in some members of Bispira (Capa 2008) and Branchiomma (e.g. Knight-Jones 1994; Licciano and Giangrande 2008). Although Sabella has always been considered as having dorsal collar margins separated by a distinct gap, it should be treated as having them fused to the faecal groove, with conspicuous dorsal notches and no pockets (Capa 2008; Fig. c). Sabellastarte, Sabella and Pseudopotamilla typically have latero-dorsal notches and pockets present (Fig. 1i, l), whereas Bispira, Branchiomma and Stylomma (included herein) have smooth dorsal margins that are widely separated.
Char. 16–20 Thorax
-
16
Thoracic chaetiger number: (0) fixed to eight; (1) lower than eight; (2) higher than eight.
-
17
Inter-ramal eyespots: (0) absent; (1) present.
-
18
Thoracic ventral shields: (0) all of equal width; (1) decreasing in size posteriorly; (2) first noticeably longer than others.
-
19
Shape of anterior margin of first ventral shield: (0) M-shaped; (1) W-shaped; (2) straight.
-
20
Thoracic neuropodial tori and ventral shields: (0) all in contact; (1) the anterior separated, the posterior in contact; (2) all separated.
Most sabellids have eight thoracic chaetigers, but in some taxa that reproduce by scissiparity this number can vary due to inconsistencies during the regeneration of segments (Capa 2007, 2008; Knight-Jones and Bowden 1984; Knight-Jones and Giangrande 2003; Tovar-Hernández and Knight-Jones 2006). In the ingroup, interramal eyespots are present on both thorax and abdomen (Fig. 1d, n), a synapomorphy of the clade (Fitzhugh 1989; Fitzhugh and Rouse 1999). The ventral shields, easily distinguished in the terminals included, can be similar in size along the thorax as in Branchiomma, Pseudobranchiomma and Pseudopotamilla, or decrease posteriorly as in Sabella or Sabellastarte (Fig. 1b, h, k). The anterior margin of the first ventral shield can vary in shape, herein classified as straight, M-shaped (when the midline notch dips in the margin) or W-shaped (when the border of the ventral shield extends upwards in the middle to contact the notch) (Capa 2008). In members of Sabellastarte all thoracic tori are in contact with the ventral shields (Fig. 1h, k), whereas they are all separated in Pseudopotamilla. This feature shows intra-generic variation in other members of the ingroup, and some species have separated or joint tori and ventral shields (Capa 2007, 2008; Knight-Jones 1994; Nogueira and Knight-Jones 2002).
Char. 21–25 Thoracic chaetae
-
21
Rows of teeth over the main fang: (0) maximally five rows; (1) many rows.
-
22
Base of thoracic uncini: (0) concave; (1) straight line.
-
23
Length of thoracic uncini handle: (0) short; (1) medium; (2) long.
-
24
Companion chaetae: (0) absent; (1) present.
-
25
Shape of companion chaetae: (0) with symmetrical membrane; (1) with asymmetrical membrane.
Traditionally the chaetae of sabellids, especially the inferior thoracic notochaetae, have been used as a diagnostic feature for genera and species (Fitzhugh 1989; Knight-Jones and Perkins 1998). Spine-like inferior thoracic notochaetae are characteristic of the ingroup, whereas in the outgroup the chaetae are paleate. These chaetae are homogeneous among species of Sabellastarte. Similarly, the shape of uncini is commonly used as a diagnostic feature. They show variability in the number of rows and size of teeth above the main fang in Bispira, Branchiomma and Pseudobranchiomma. These teeth are large and arranged in a few rows (five or fewer), while Sabella and Sabellastarte show smaller teeth and numerous rows. The development of the breast of thoracic uncini has been incorporated in previous phylogenetic analyses of Sabellidae (Capa 2007, 2008; Fitzhugh 1989; Fitzhugh and Rouse 1999; Rouse and Fitzhugh 1994), which differentiated a plesiomorphic narrow breast condition from an apomorphic well developed breast. Handle length has also been considered as informative (e.g. Capa 2007; 2008; Fitzhugh 1989; Fitzhugh and Rouse 1999), being classified as short when the handles are shorter than the distance between the tip of the main fang and the base of the breast, as medium when the length is anywhere up to double that distance, and as long when it is even longer. The base of the uncini and handle can be straight, concave or occasionally convex. Members of Branchiomma and Pseudobranchiomma have a concave base, whereas Bispira show a straight base. In members of Sabellastarte, however, this character can be inconsistent, even among members of the same population (Fig. 8). The uncini of members of Pseudopotamilla have longer handles than in the ingroup, and the base is straight.
Char. 26–28 Abdomen
-
26
Abdominal neuropodia: (0) transverse ridges; (1) conical lobes.
-
27
Dorsal notopodial eyes in abdominal chaetigers: (0) absent; (1) present.
-
28
Neuropodial eyespots in abdominal chaetigers: (0) absent; (1) present.
The neuropodium in abdominal chaetigers is a transverse ridge in Pseudopotamilla and a conical lobe in the ingroup (Fig. 1d). As described for the thorax, the genera included in the ingroup show interramal eyespots in the abdomen (Capa 2008; Fitzhugh 1989; Fitzhugh and Rouse 1999). Some individuals of Sabellastarte have spots on the dorsum of the abdominal notopodia and on the venter of neuropodia; although no photoreceptor has been observed, the spots seem to be similar to those found between noto- and neuropodia, meaning that there could be three eyespots on each parapodium (Figs. 5c, f, 6c, f, i, k, 7 l and 8).
a–cSabellastarte from Kota Kinabalu, Malaysia; AM W.32025. a Anterior thoracic segments, ventral view. b Anterior thoracic segments, dorsal view. c Mid abdominal segments, lateral view. d–fSabellastarte from Oahu, Hawaii; AM W.31843. d Anterior thoracic segments, ventral view. e Anterior thoracic segments, dorsal view. f Mid abdominal segments, lateral view. g–iSabellastarte from Saipan, Northern Marianas Islands; AM W.31847. g Anterior thoracic segments, ventral view. h Anterior thoracic segments, dorsal view. i Mid abdominal segments, lateral view. j–lSabellastarte from Karachi, Pakistan; AM W.31824. j Anterior thoracic segments, ventral view. (K) Anterior thoracic segments, dorsal view. l Mid abdominal segments, lateral view
a–cSabellastarte australiensis from Sydney, New South Wales, Australia. a Anterior thoracic segments, ventral view. b Anterior thoracic segments, dorsal view. c Mid abdominal segments, lateral view. d–fSabellastarte from Mallacoota, Victoria, Australia, MV F.108859. d Anterior thoracic segments, ventral view. e Anterior thoracic segments, dorsal view. f Mid abdominal segments, lateral view.g–iSabellastarte from Kimberleys, Western Australia. g Anterior thoracic segments, ventral view. h Anterior thoracic segments, dorsal view. i Mid abdominal segments, lateral view. j–lSabellastarte from Darwin, Northern Territory, Australia, AM W.31844, j Anterior thoracic segments, ventral view. k Anterior thoracic segments, dorsal view. l Mid abdominal segments, lateral view
Line drawings of pairs of uncini from various specimens; respective left drawing: uncinus from seventh thoracic chaetiger, right drawing: uncinus from a mid-abdominal chaetiger. a, b Veracruz, Mexico; AM W.31846. c, d Sagami Bay, Japan; AM W.31845. e, f Karachi, Pakistan; AM W.31823. g, h Kota Kinabalu, Malaysia; AM W.30385. i, j Saipan, Northern Marianas Islands; AM W.31847. k, l Pago Pago, American Samoa; AM W.32479. m, n Port Jackson, New South Wales, Australia; AM G.2045 (?type). o, p Port Jackson, New South Wales, Australia; AM W.4280. q, r Port Phillip Bay, Victoria, Australia; AM W.11967. s, t Port Phillip Bay, Victoria, Australia; MV.F.108866. u, v Kimberleys, Western Australia; AM Ex W.202945. w, x Darwin, Northern Territory, Australia; NTM W.17197. Scales: a–l, u–x: 1 μm; m–t: 4 μm
Char. 29, 30 Abdominal chaetae
-
29
Superior abdominal chaetae: (0) in straight line; (1) in C-shaped arrangement.
-
30
Number or rows of inferior abdominal chaetae: (0) one; (1) two; (2) three; (3) higher than three.
The ingroup taxa are characterized by having the superior abdominal chaetae displayed in a C-shape or a spiral in members of Sabella (Fig. 1d; see also Capa 2008). In their revision of the genus, Knight-Jones and Mackie (2003) considered the number of chaetae in mature specimens as diagnostic for some Sabellastarte species. Due to our observation that the number is quite variable among the observed specimens, the number of rows of chaetae has been taken into account instead, and it has been counted on the anterior abdominal segments.
Char. 31, 32 Pygidium
-
31
Pygidial eyes: (0) absent; (1) present.
-
32
Pygidium: (0) bilobed; (1) rim; (2) papilla.
The shape of the pygidium and the presence of pygidial eyespots (Fig. 1 n) have been included in the present study, but some museum specimens were so damaged that these characters could be scored as question marks only.
Excluded characters
Certain morphological characters that show variation among populations have been excluded from the morphological data matrix even though they were included in species descriptions in the past. For example, the length of the branchial crown relative to body length has been used in Sabellastarte species descriptions (Knight-Jones and Mackie 2003), even though it varies during regular growth of the animal as well as during regeneration after damage. The outer margins of radioles have been considered as rounded or subrectangular in a previous cladistic analysis (Fitzhugh 1989), but objectively their shape is hard to assign to discrete categories, and variation has been observed among radioles from a single specimen or even along their individual length. The radiolar pinnules of most sabellids, especially in large species, are constant in length along the radiole, diminishing in size at the distal end. However, some Sabellastarte show different lengths of pinnules along the radioles, giving an undulating appearance to their margins, but this attribute is hard to perceive in fixed specimens with curled pinnules, therefore has not been included. The relation between thorax length and width was used to distinguish species of Sabellastarte (Knight-Jones and Mackie 2003), but the corresponding measurement results can depend on fixation procedures (Costa-Paiva et al. 2007). The number of chaetae in Sabellastarte thoracic parapodia, a character used to describe new species (Knight-Jones and Mackie 2003), can reach higher numbers than in any other sabellid. However, the number of thoracic chaetae varies during growth and development, thus has been omitted here. The width of the ‘knee’ or ‘hood’ relative to the ‘shaft’ or ‘core’ of the thoracic chaetae was also used in species descriptions (Knight-Jones and Mackie 2003). This character shows intra-specific variation, even among chaetae on a single parapodium, and as a quasi-cylindrical structure varies with the observation angle.
Rights and permissions
About this article
Cite this article
Capa, M., Bybee, D.R. & Bybee, S.M. Establishing species and species boundaries in Sabellastarte Krøyer, 1856 (Annelida: Sabellidae): an integrative approach. Org Divers Evol 10, 351–371 (2010). https://doi.org/10.1007/s13127-010-0033-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-010-0033-z