Abstract
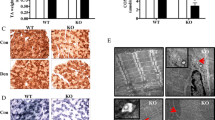
The abundance, morphology, and functional properties of mitochondria become altered in response to denervation. To gain insight into the regulation of this process, mitochondrial enzyme activities and gene expression involved in mitochondrial biogenesis and dynamics in mouse gastrocnemius muscle was investigated. Sciatic nerve transactions were performed on mice, and then gastrocnemius muscles were isolated at days 5 and 30 after surgery. Muscle weight was decreased significantly by 15% and 62% at days 5 and 30 after surgery, respectively. The activity of citrate synthase, a marker of oxidative enzyme, was reduced significantly by 31% and 53% at days 5 and 30, respectively. Enzyme histochemical analysis revealed that subsarcolemmal mitochondria were largely lost than intermyofibrillar mitochondria at day 5, and this trend was further progressed at day 30 after surgery. Expression levels of peroxisome proliferator-activated receptor, γ coactivator 1 (PGC-1)α, estrogen-related receptor α (ERRα), and mitofusin 2 were down-regulated throughout the experimental period, whereas those of PGC-1β, PRC, nuclear respiratory factor (NRF)-1, NRF-2, TFAM, and Lon protease were down-regulated at day 30 after surgery. These results suggest that PGC-1α, ERRα, and mitofusin 2 may be important factors in the process of denervation-induced mitochondrial adaptation. In addition, other PGC-1 family of transcriptional coactivators and DNA binding transcription factors may also contribute to mitochondrial adaptation after early response to denervation.




Similar content being viewed by others
References
Adhihetty PJ, O’Leary MF, Chabi B, Wicks KL, Hood DA (2007) Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 102:1143–1151
Agbulut O, Vignaud A, Hourde C, Mouisel E, Fougerousse F, Butler-Browne GS, Ferry A (2009) Slow myosin heavy chain expression in the absence of muscle activity. Am J Physiol 296:C205–C214
Andersson U, Scarpulla RC (2001) Pgc-1-related coactivator, a novel, serum-inducible coactivator of nuclear respiratory factor 1-dependent transcription in mammalian cells. Mol Cell Biol 21:3738–3749
Bendt AK, Burkovski A, Schaffer S, Bott M, Farwick M, Hermann T (2003) Towards a phosphoproteome map of Corynebacterium glutamicum. Proteomics 3:1637–1646
Booth FW (1982) Effect of limb immobilization on skeletal muscle. J Appl Physiol 52:1113–1118
Borisov AB, Huang SK, Carlson BM (2000) Remodeling of the vascular bed and progressive loss of capillaries in denervated skeletal muscle. Anat Rec 258:292–304
Bota DA, Davies KJ (2002) Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol 4:674–680
Brault JJ, Jespersen JG, Goldberg AL (2010) Peroxisome proliferator-activated receptor gamma coactivator 1alpha or 1beta overexpression inhibits muscle protein degradation, induction of ubiquitin ligases, and disuse atrophy. J Biol Chem 285:19460–19471
Bruni F, Polosa PL, Gadaleta MN, Cantatore P, Roberti M (2010) Nuclear respiratory factor 2 induces the expression of many but not all human proteins acting in mitochondrial DNA transcription and replication. J Biol Chem 285:3939–3948
Carrier JC, Deblois G, Champigny C, Levy E, Giguère V (2004) Estrogen-related receptor alpha (ERRalpha) is a transcriptional regulator of apolipoprotein A-IV and controls lipid handling in the intestine. J Biol Chem 279:52052–52058
Cartoni R, Léger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Dériaz O, Zorzano A, Gobelet C, Kralli A, Russell AP (2005) Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 567:349–358
Chabi B, Adhihetty PJ, O’Leary MF, Menzies KJ, Hood DA (2009) Relationship between Sirt1 expression and mitochondrial proteins during conditions of chronic muscle use and disuse. J Appl Physiol 107:1730–1735
Chalmers GR, Edgerton VR (1989) Single motoneuron succinate dehydrogenase activity. J Histochem Cytochem 37:1107–1114
Chen H, Chan DC (2005) Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet 14:R283–R289
Fernández-Silva P, Enriquez JA, Montoya J (2003) Replication and transcription of mammalian mitochondrial DNA. Exp Physiol 88:41–56
Gardner PR, Nguyen DD, White CW (1994) Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci 91:12248–12252
Glass DJ (2003) Molecular mechanisms modulating muscle mass. Trends Mol Med 9:344–350
Gleyzer N, Vercauteren K, Scarpulla RC (2005) Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25:1354–1366
Goldspink DF (1976) The effects of denervation on protein turnover of rat skeletal muscle. Biochem J 156:71–80
Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM (2007) Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 282:30014–30021
Henriksen EJ, Rodnick KJ, Mondon CE, James DE, Holloszy JO (1991) Effect of denervation or unweighting on GLUT-4 protein in rat soleus muscle. J Appl Physiol 70:2322–2327
Hock MB, Kralli A (2009) Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 71:177–203
Hood DA (2001) Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 90:1137–1157
Hoppeler H (1986) Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med 7:187–204
Huo L, Scarpulla RC (2001) Mitochondrial DNA instability and peri-implantation lethality associated with targeted disruption of nuclear respiratory factor 1 in mice. Mol Cell Biol 21:644–654
Huss JM, Torra IP, Staels B, Giguère V, Kelly DP (2004) Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 24:9079–9091
Irintchev A, Draguhn A, Wernig A (1990) Reinnervation and recovery of mouse soleus muscle after long-term denervation. Neuroscience 39:231–243
Jato-Rodriguez J, Liang CR, Lin CH, Hudson AJ, Strickland KP (1975) Comparison of the intermediary metabolism of fatty acids in denervated and dystrophic murine skeletal muscle. J Neurol Neurosurg Psychiatry 38:1083–1089
Joffe M, Savage N, Isaacs H (1981) Ca2+-uptake properties of two populations of mitochondria from normal and denervated rat soleus muscle. Biochem J 200:671–677
Kirby DM, Thorburn DR, Turnbull DM, Taylor RW (2007) Biochemical assays of respiratory chain complex activity. Methods Cell Biol 80:93–119
Kostrominova TY, Dow DE, Dennis RG, Miller RA, Faulkner JA (2005) Comparison of gene expression of 2-mo denervated, 2-mo stimulated-denervated, and control rat skeletal muscles. Physiol Genomics 22:227–243
Kraft CS, LeMoine CM, Lyons CN, Michaud D, Mueller CR, Moyes CD (2006) Control of mitochondrial biogenesis during myogenesis. Am J Physiol 290:C1119–C1127
Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18:231–236
Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly YM, Storlien L, Strömstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig A (2006) Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4:e369
Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP (2005) PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101
Liesa M, Borda-d’Agua B, Medina-Gómez G, Lelliott CJ, Paz JC, Rojo M, Palacín M, Vidal-Puig A, Zorzano A (2008) Mitochondrial fusion is increased by the nuclear coactivator PGC-1beta. PLoS ONE 3:e3613
Liesa M, Palacín M, Zorzano A (2009) Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89:799–845
Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM (2002) Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem 277:1645–1648
Lu DX, Huang SK, Carlson BM (1997) Electron microscopic study of long-term denervated rat skeletal muscle. Anat Rec 248:355–365
Masuyama M, Iida R, Takatsuka H, Yasuda T, Matsuki T (2005) Quantitative change in mitochondrial DNA content in various mouse tissues during aging. Biochim Biophys Acta 1723:302–308
Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H (2007) Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol 293:R1159–R1168
Nachlas MM, Tsou KC, DeSousa E, Cheng CS, Seligman AM (1957) Cytochemical demonstration of succinic dehydrogenase by the use of the new p-nitrophenil substituted ditetrazole. J Histochem Cytochem 5:420–436
Nikawa T, Ishidoh K, Hirasaka K, Ishihara I, Ikemoto M, Kano M, Kominami E, Nonaka I, Ogawa T, Adams GR, Baldwin KM, Yasui N, Kishi K, Takeda S (2004) Skeletal muscle gene expression in space-flown rats. FASEB J 18:522–524
Ongwijitwat S, Liang HL, Graboyes EM, Wong-Riley MT (2006) Nuclear respiratory factor 2 senses changing cellular energy demands and its silencing down-regulates cytochrome oxidase and other target gene mRNAs. Gene 374:39–49
Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, Palacin M, Zorzano A (2005) The Charcot–Marie–Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet 14:1405–1415
Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M (2010) Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J 29:1774–1785
Ryan MT, Hoogenraad NJ (2007) Mitochondrial-nuclear communications. Annu Rev Biochem 76:701–722
Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL (2007) Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21:140–155
Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM (2006) PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci 103:16260–16265
Scarpulla RC (2008) Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88:611–638
Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A (2003) The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha). J Biol Chem 278:9013–9018
Siu PM, Alway SE (2005) Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol 565:309–323
Soriano FX, Liesa M, Bach D, Chan DC, Palacín M, Zorzano A (2006) Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 55:1783–1791
Srere PA (1969) Citrate synthase. Methods Enzymol 13:3–5
Suliman HB, Carraway MS, Welty-Wolf KE, Whorton AR, Piantadosi CA (2003) Lipopolysaccharide stimulates mitochondrial biogenesis via activation of nuclear respiratory factor-1. J Biol Chem 278:41510–41518
Talmadge RJ (2000) Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve 23:661–679
Thomason DB, Booth FW (1990) Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol 68:1–12
Tomáska L, Nosek J, Kucejová B (2001) Mitochondrial single-stranded DNA-binding proteins: in search for new functions. Biol Chem 382:179–186
van den Bosch BJ, van den Burg CM, Schoonderwoerd K, Lindsey PJ, Scholte HR, de Coo RF, van Rooij E, Rockman HA, Doevendans PA, Smeets HJ (2005) Regional absence of mitochondria causing energy depletion in the myocardium of muscle LIM protein knockout mice. Cardiovasc Res 65:411–418
Vercauteren K, Gleyzer N, Scarpulla RC (2009) Short hairpin RNA-mediated silencing of PRC (PGC-1-related coactivator) results in a severe respiratory chain deficiency associated with the proliferation of aberrant mitochondria. J Biol Chem 284:2307–2319
Wagatsuma A, Osawa T (2006) Time course of changes in angiogenesis-related factors in denervated muscle. Acta Physiol 187:503–509
Wagatsuma A, Kotake N, Kawachi T, Shiozuka M, Yamada S, Matsuda R Mitochondrial adaptations in skeletal muscle to hindlimb unloading. Mol Cell Biochem, doi:10.1007/s11010-010-0677-1
Wicks KL, Hood DA (1991) Mitochondrial adaptations in denervated muscle: relationship to muscle performance. Am J Physiol 260:C841–C850
Wiwi CA, Gupte M, Waxman DJ (2004) Sexually dimorphic P450 gene expression in liver-specific hepatocyte nuclear factor 4alpha-deficient mice. Mol Endocrinol 18:1975–1987
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Yakes FM, Van Houten B (1997) Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci 94:514–519
Acknowledgement
This research was supported by the MEXT (The Ministry of Education, Culture, Sports, Science and Technology; Grant-in Aid for Scientific Research (C), 22500658), Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagatsuma, A., Kotake, N., Mabuchi, K. et al. Expression of nuclear-encoded genes involved in mitochondrial biogenesis and dynamics in experimentally denervated muscle. J Physiol Biochem 67, 359–370 (2011). https://doi.org/10.1007/s13105-011-0083-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-011-0083-5