Abstract
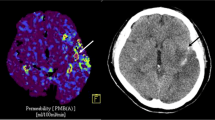
Permeability imaging might add valuable information in the risk assessment of hemorrhagic transformation. This study evaluates the predictive value of blood–brain barrier permeability (BBBP) measurements extracted from dynamic contrast-enhanced MRI for hemorrhagic transformation in ischemic stroke. Spontaneously hypertensive and Wistar rats with 2 h filament occlusion of the right MCA underwent MRI during occlusion, at 4 and 24 h post reperfusion. BBBP was imaged by DCE imaging and quantified by Patlak analysis. Cresyl-violet staining was used to characterize hemorrhage in sacrificed rats at 24 h, immediately following the last imaging study. BBBP changes were evaluated at baseline, 4 and 24 h after reperfusion. Receiver-operating characteristic (ROC) analysis was performed to determine the most accurate BBBP threshold to predict hemorrhagic transformation. In animals showing macroscopic hemorrhage at 24 h, 95th BBBP percentile values ipsilateral were 0.323 [0.260, 0.387], 0.685 [0.385, 0.985], and 0.412 [0.210, 0.613] ml/min·100 g (marginal mean [95%CI]) during occlusion, at 4 and 24 h post reperfusion, respectively. The BBBP values on the infarcted and contralateral side were significantly different at 4 (p = 0.034) and 24 h post reperfusion (p = 0.031). The predictive value of BBBP in terms of macroscopic hemorrhage was highest 4 h after reperfusion (ROC area under the curve = 84 %) with a high negative predictive value (98.3 %) and limited positive predictive value (14.9 %) for a threshold of 0.35 ml/min·100g. Altered BBBP is a necessary but not sufficient condition to cause hemorrhagic transformation in rats with an infarct. Further research is needed to identify those additional risk factors that are required for hemorrhagic transformation to develop in the setting of ischemic stroke.



Similar content being viewed by others
References
Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107(4):598–603.
Demchuk AM, Morgenstern LB, Krieger DW, Linda Chi T, Hu W, Wein TH, et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30(1):34–9.
Castellanos M, Serena J. Applicability of biomarkers in ischemic stroke. Cerebrovasc Dis. 2007;24 Suppl 1:7–15. doi:10.1159/000107374.
Butcher K, Christensen S, Parsons M, De Silva DA, Ebinger M, Levi C, et al. Postthrombolysis blood pressure elevation is associated with hemorrhagic transformation. Stroke. 2010;41(1):72–7. doi:10.1161/STROKEAHA.109.563767.
Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001;32(2):438–41.
Selim M, Fink JN, Kumar S, Caplan LR, Horkan C, Chen Y, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke. 2002;33(8):2047–52.
Lansberg MG, Thijs VN, Bammer R, Kemp S, Wijman CA, Marks MP, et al. Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007;38(8):2275–8. doi:10.1161/STROKEAHA.106.480475.
Campbell BC, Christensen S, Butcher KS, Gordon I, Parsons MW, Desmond PM, et al. Regional very low cerebral blood volume predicts hemorrhagic transformation better than diffusion-weighted imaging volume and thresholded apparent diffusion coefficient in acute ischemic stroke. Stroke. 2010;41(1):82–8. doi:10.1161/STROKEAHA.109.562116.
Knight RA, Barker PB, Fagan SC, Li Y, Jacobs MA, Welch KM. Prediction of impending hemorrhagic transformation in ischemic stroke using magnetic resonance imaging in rats. Stroke. 1998;29(1):144–51.
Neumann-Haefelin C, Brinker G, Uhlenkuken U, Pillekamp F, Hossmann KA, Hoehn M. Prediction of hemorrhagic transformation after thrombolytic therapy of clot embolism: an MRI investigation in rat brain. Stroke. 2002;33(5):1392–8.
Kassner A, Roberts T, Taylor K, Silver F, Mikulis D. Prediction of hemorrhage in acute ischemic stroke using permeability MR imaging. AJNR Am J Neuroradiol. 2005;26(9):2213–7.
Bang OY, Buck BH, Saver JL, Alger JR, Yoon SR, Starkman S, et al. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann Neurol. 2007;62(2):170–6. doi:10.1002/ana.21174.
Bisdas S, Hartel M, Cheong LH, Koh TS. Detection of early vessel leakiness in acute ischemic stroke using computed tomography perfusion may indicate hemorrhagic transformation. Acta Radiol. 2007;48(3):341–4. doi:10.1080/02841850601182170.
Aviv RI, d’Esterre CD, Murphy BD, Hopyan JJ, Buck B, Mallia G, et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology. 2009;250(3):867–77. doi:10.1148/radiol.2503080257.
Hom J, Dankbaar JW, Soares BP, Schneider T, Cheng SC, Bredno J, et al. Blood–brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol. 2011;32(1):41–8. doi:10.3174/ajnr.A2244.
Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28(3):229–44. doi:10.1385/MN:28:3:229.
Kassner A, Mandell DM, Mikulis DJ. Measuring permeability in acute ischemic stroke. Neuroimaging Clin N Am. 2011;21(2):315–25. doi:10.1016/j.nic.2011.01.004.
Rouchaud A, Mazighi M, Labreuche J, Meseguer E, Serfaty JM, Laissy JP et al. (2011) Outcomes of mechanical endovascular therapy for acute ischemic stroke: a clinical registry study and systematic review. Stroke. 42(5):1289–94. doi:10.1161/STROKEAHA.110.599399.
Hoffmann A, Bredno J, Wendland MF, Derugin N, Hom J, Schuster T, et al. Validation of in vivo magnetic resonance imaging blood–brain barrier permeability measurements by comparison with gold standard histology. Stroke. 2011. doi:10.1161/STROKEAHA.110.597997.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91.
Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, et al. Comparative overview of brain perfusion imaging techniques. Stroke. 2005;36(9):e83–99. doi:10.1161/01.STR.0000177884.72657.8b.
Wintermark M, Maeder P, Thiran JP, Schnyder P, Meuli R. Quantitative assessment of regional cerebral blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models. Eur Radiol. 2001;11(7):1220–30.
Ladurner G, Zilkha E, Iliff D, du Boulay GH, Marshall J. Measurement of regional cerebral blood volume by computerized axial tomography. J Neurol Neurosurg Psychiatry. 1976;39(2):152–8.
Axel L. Tissue mean transit time from dynamic computed tomography by a simple deconvolution technique. Investig Radiol. 1983;18(1):94–9.
George Paxinos CW. The rat brain in stereotaxic coordinates—the new coronal set, fifth edition. Burlington: Elsevier Academic Press; 2005.
Ding G, Nagesh V, Jiang Q, Zhang L, Zhang ZG, Li L, et al. Early prediction of gross hemorrhagic transformation by noncontrast agent MRI cluster analysis after embolic stroke in rat. Stroke. 2005;36(6):1247–52. doi:10.1161/01.STR.0000166199.10017.c5.
Lin K, Kazmi KS, Law M, Babb J, Peccerelli N, Pramanik BK. Measuring elevated microvascular permeability and predicting hemorrhagic transformation in acute ischemic stroke using first-pass dynamic perfusion CT imaging. AJNR Am J Neuroradiol. 2007;28(7):1292–8. doi:10.3174/ajnr.A0539.
Ding G, Jiang Q, Li L, Zhang L, Gang Zhang Z, Ledbetter KA, et al. Detection of BBB disruption and hemorrhage by Gd-DTPA enhanced MRI after embolic stroke in rat. Brain Res. 2006;1114(1):195–203. doi:10.1016/j.brainres.2006.07.116.
Hom J, Dankbaar JW, Soares BP, Schneider T, Cheng SC, Bredno J et al. (2011) Blood–brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol. 32(1):41–8. doi:10.3174/ajnr.A2244.
Molina CA, Alvarez-Sabin J, Montaner J, Abilleira S, Arenillas JF, Coscojuela P, et al. Thrombolysis-related hemorrhagic infarction: a marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke. 2002;33(6):1551–6.
Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30(11):2280–4.
Kim JH, Bang OY, Liebeskind DS, Ovbiagele B, Kim GM, Chung CS, et al. Impact of baseline tissue status (diffusion-weighted imaging lesion) versus perfusion status (severity of hypoperfusion) on hemorrhagic transformation. Stroke. 2010;41(3):e135–42. doi:10.1161/STROKEAHA.109.563122.
Chan PH. Oxygen radicals in focal cerebral ischemia. Brain Pathol. 1994;4(1):59–65.
Gursoy-Ozdemir Y, Can A, Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35(6):1449–53. doi:10.1161/01.STR.0000126044.83777.f4.
Dijkhuizen RM, Asahi M, Wu O, Rosen BR, Lo EH. Rapid breakdown of microvascular barriers and subsequent hemorrhagic transformation after delayed recombinant tissue plasminogen activator treatment in a rat embolic stroke model. Stroke. 2002;33(8):2100–4.
Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi:10.1056/NEJMoa0804656.
Pillai DR, Dittmar MS, Baldaranov D, Heidemann RM, Henning EC, Schuierer G, et al. Cerebral ischemia-reperfusion injury in rats—a 3 T MRI study on biphasic blood–brain barrier opening and the dynamics of edema formation. J Cereb Blood Flow Metab. 2009;29(11):1846–55. doi:10.1038/jcbfm.2009.106.
Chen CH, Toung TJ, Sapirstein A, Bhardwaj A. Effect of duration of osmotherapy on blood–brain barrier disruption and regional cerebral edema after experimental stroke. J Cereb Blood Flow Metab. 2006;26(7):951–8. doi:10.1038/sj.jcbfm.9600248.
Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood–brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739(1–2):88–96.
Albayrak S, Zhao Q, Siesjo BK, Smith ML. Effect of transient focal ischemia on blood–brain barrier permeability in the rat: correlation to cell injury. Acta Neuropathol. 1997;94(2):158–63.
Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM. Biphasic opening of the blood–brain barrier following transient focal ischemia: effects of hypothermia. Can J Neurol Sci. 1999;26(4):298–304.
Strbian D, Durukan A, Pitkonen M, Marinkovic I, Tatlisumak E, Pedrono E, et al. The blood–brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience. 2008;153(1):175–81. doi:10.1016/j.neuroscience.2008.02.012.
Jiang Q, Zhang RL, Zhang ZG, Knight RA, Ewing JR, Ding G, et al. Magnetic resonance imaging characterization of hemorrhagic transformation of embolic stroke in the rat. J Cereb Blood Flow Metab. 2002;22(5):559–68. doi:10.1097/00004647-200205000-00007.
Bang OY, Saver JL, Alger JR, Shah SH, Buck BH, Starkman S, et al. Patterns and predictors of blood–brain barrier permeability derangements in acute ischemic stroke. Stroke. 2009;40(2):454–61. doi:10.1161/STROKEAHA.108.522847.
Kassner A, Roberts TP, Moran B, Silver FL, Mikulis DJ. Recombinant tissue plasminogen activator increases blood–brain barrier disruption in acute ischemic stroke: an MR imaging permeability study. AJNR Am J Neuroradiol. 2009;30(10):1864–9. doi:10.3174/ajnr.A1774.
Fukuda S, del Zoppo GJ. Models of focal cerebral ischemia in the nonhuman primate. ILAR J. 2003;44(2):96–104.
Sources of Funding
This study was supported by a seed grant from the University of California San Francisco, Department of Radiology and Biomedical Imaging and P01 NS044155 (H.S., W.L.Y.).
Disclosures
J.B. is an employee of Philips Healthcare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 166 kb)
Rights and permissions
About this article
Cite this article
Hoffmann, A., Bredno, J., Wendland, M.F. et al. MRI Blood–Brain Barrier Permeability Measurements to Predict Hemorrhagic Transformation in a Rat Model of Ischemic Stroke. Transl. Stroke Res. 3, 508–516 (2012). https://doi.org/10.1007/s12975-012-0212-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-012-0212-7