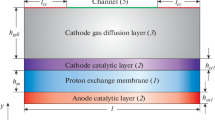
Abstract
We report the most general analytical solutions to a macrohomogeneous model for performance of the cathode catalyst layer (CCL) in PEM fuel cell. Equations for the shapes of local parameters through the CCL thickness and for the CCL polarization curve are derived for the regime of mixed oxygen- and proton-transport limitations. In the limit of ideal oxygen transport, an equation for the CCL polarization curve valid in the whole range of currents is obtained. Good agreement between analytical and exact numerical results is demonstrated.




Similar content being viewed by others
Notes
A reasonable estimate for the derivative is \({\partial {\tilde{j}}}/{\partial {\tilde{x}}} \simeq \tilde{j}_0\). With \(\tilde{c} \leq 1\), we see that in Eq. 10, unity under the square root can be neglected if \(\tilde{j}_0 > 1/\varepsilon^2\). In PEMFCs, ε ≃ 103; with the characteristic current density j * ≃ 1 A cm − 2, this means that this approximation works well for current densities above 10 − 3 mA cm − 2.
Uniform over the cell surface, GDL flooding or membrane drying could be taken into account by simple scaling of the parameter D b or R, respectively.
References
Adzic RR et al (2007) Top Catal 46:249
Bernardi DM, Verbrugge MW (1991) AIChE J 37:1151
Springer TE, Zawodzinski TA, Gottesfeld S (1991) J Electrochem So 138:2334
Frumkin AN, Bagotsky VS, Iofa ZA, Kabanov BN (1952) Kinetics of electrode processes. Izd. Mosk. Univ., Moscow
Perry ML, Newman J, Cairns EJ (1998) J Electrochem Soc 145:5
Eikerling M, Kornyshev AA (1998) J Electroanal Chem 453:89
Wang Y, Feng X (2008) J Electrochem Soc 155:B1289
Wang Y, Feng X (2009) J Electrochem Soc 156:B403
Kulikovsky AA (2010) Electrochim Acta 55:6391
Eikerling M (2006) J Electrochem Soc 153:E58
Ioselevich AS, Kornyshev AA (2001) Fuel Cells 1:40
Kulikovsky AA (2010) Analytical modelling of fuel cells. Elsevier, Amsterdam
Kulikovsky AA (2009) Electrochem Electochem Solid State Lett 12:B53
Kulikovsky AA (2012) J Electroanal Chem 669:28
Shen J et al (2011) J Power Sources 96:674
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix
Here, we report the details of the derivation of Eq. 15. Integrating Eq. 13 once, we get
where β is constant. Equation 30 can, in principle, further be integrated; however, this leads to rather cumbersome expressions. Fortunately, smallness of γ allows us to employ asymptotic technique. We write
where \(\tilde{j}^0\) is the leading-order solution and \(\tilde{j}^1\) is the first-order correction. Substituting Eq. 31 into Eq. 30, collecting the terms with like powers of γ and neglecting the terms with γ 2, we get equations for \(\tilde{j}^0\) and \(\tilde{j}^1\):
The solution to Eq. 32 is
With this \(\tilde{j}^0\), the solution to Eq. 33 reads
The oxygen concentration and parameter γ can now be calculated using the leading-order solution \(\tilde{j}^0\) in equation for \(\tilde{c}\). Substituting \(\tilde{j}=\tilde{j}^0\) into Eq. 8, we get
where \(\tilde{c}_1\) is the oxygen concentration at the CCL/GDL interface. Solving this equation, we find
Calculating \({\partial {\ln\tilde{c}}}/{\partial {\tilde{x}}}\) and setting \(\tilde{x}=1\) in the resulting expression, we find
Thus, the analysis above is valid provided that \(\tilde{j}_0/(\tilde{D}\tilde{c}_1) \ll 1\).
The approximate solution to Eq. 13 is given by Eq. 31 with \(\tilde{j}^0\), \(\tilde{j}^1\), and γ given by Eqs. 34, 35, and 38, respectively. Parameter β can be found from the condition \(\tilde{j}(0) = \tilde{j}_0\); with Eq. 31, this leads to the following equation:
The shape of local overpotential can now be calculated from Eq. 6:
Using here Eqs. 31, 34, 35, and 37, we come up with Eq. 15.
Nomenclature
- \(\tilde{~}\) :
-
Marks dimensionless variables
- b :
-
Tafel slope (V)
- c :
-
Oxygen molar concentration (mol cm − 3)
- c ref :
-
Reference oxygen molar concentration (mol cm − 3)
- D :
-
Oxygen diffusion coefficient in the CCL (cm2 s − 1)
- D * :
-
Characteristic oxygen diffusivity (cm2 s − 1), Eq. 5
- D b :
-
Oxygen diffusion coefficient in the GDL (cm2 s − 1)
- F :
-
Faraday constant
- j 0 :
-
Cell current density (A cm − 2)
- j :
-
Local proton current density in the CCL (A cm − 2)
- j * :
-
Characteristic current density (A cm − 2), Eq. 5
- i * :
-
Volumetric exchange current density (A cm − 3)
- l b :
-
GDL thickness (cm)
- l t :
-
Catalyst layer thickness (cm)
- n :
-
Number of electrons transferred in the half-cell reaction
- x :
-
Coordinate across the CCL (cm)
Subscripts
- 0:
-
Membrane/CCL interface
- 1:
-
CCL/GDL interface
- b :
-
GDL
- t :
-
Catalyst layer
- *:
-
Characteristic value
Greek
Rights and permissions
About this article
Cite this article
Kulikovsky, A.A. Catalyst Layer Performance in PEM Fuel Cell: Analytical Solutions. Electrocatalysis 3, 132–138 (2012). https://doi.org/10.1007/s12678-012-0091-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-012-0091-4