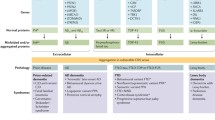
Abstract
The notion of staging in the neurodegenerative disorders is modulated by the constant and progressive loss of several aspects of brain structural integrity, circuitry, and neuronal processes. These destructive processes eventually remove individuals’ abilities to perform at sufficient and necessary functional capacity at several levels of disease severity. The classification of (a) patients on the basis of diagnosis, risk prognosis, and intervention outcome, forms the basis of clinical staging, and (b) laboratory animals on the basis of animal model of brain disorder, extent of insult, and dysfunctional expression, provides the components for the clinical staging and preclinical staging , respectively, expressing associated epidemiological, biological, and genetic characteristics. The major focus of clinical staging in the present account stems from the fundamental notions of Braak staging as they describe the course and eventual prognosis for Alzheimer’s disease, Lewy Body dementia, and Parkinson’s disease. Mild cognitive impairment, which expresses the decline in episodic and semantic memory performance below the age-adjusted normal range without marked loss of global cognition or activities of daily living, and the applications of longitudinal magnetic resonance imaging, major instruments for the monitoring of either disease progression in dementia, present important challenges for staging concepts. Although Braak notions present the essential basis for further developments, current staging conceptualizations seem inadequate to comply with the massive influx of information dealing with neurodegenerative processes in brain, advanced both under clinical realities, and discoveries in the laboratory setting. The contributions of various biomarkers of disease progression, e.g., amyloid precursor protein, and neurotransmitter system imbalances, e.g., dopamine receptor supersensitivity and interactive propensities, await their incorporation into the existing staging models thereby underlining the ongoing, dynamic feature of the staging of brain disorders.


Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer disease
- APP:
-
Amyloid precursor protein
- AS:
-
α-Synucleinopathy
- CBD:
-
Corticobasal degeneration
- CBS:
-
Corticobasal syndrome
- CIT:
-
2-β-Carboxymethoxy-3-β-(4-iodophenyl) tropane
- CSF:
-
Cerebrospinal fluid
- DA:
-
Dopamine
- DARSS:
-
Dopamine receptor supersensitivity
- DG-PET:
-
Deoxyglucose-positron emission tomography
- fMRI:
-
Functional magnetic resonance imaging
- FTD:
-
Frontotemporal dementia
- MR:
-
Magnetic resonance
- MRI:
-
Magnetic resonance imaging
- HNPCs:
-
Human neural progenitor cells
- LB:
-
Lewy body
- LBD:
-
Dementia with Lewy bodies
- MCI:
-
Mild cognitive impairment
- NFT:
-
Neurofibrillary tangle
- PD:
-
Parkinson’s disease
- PDD:
-
Parkinson’s disease dementia
- PDRP:
-
Parkinson disease motor-related pattern
- PET:
-
Positron emission tomography
- PiB:
-
Pittsburgh compound B
- RSS:
-
Receptor supersensitivity
- SMI:
-
Subjective memory impairment
- SN:
-
Substantia nigra
- SPECT:
-
Single photon emission computerized tomography
- SPECT:
-
99m Tc-HMPAO, 500 M Bq Techmetium-99 m hexamethylpropylene amine oxime
- VBM:
-
Voxel based morphometry
- VZV:
-
Varicella Zoster virus
References
Aarsland D, Ballard CG, Halliday G (2004) Are Parkinson’s disease with dementia and dementia with Lewy bodies the same entity? J Geriatr Psychiatr Neurol 17:137–145
Ahmed S, Arnold R, Thompson SA, Graham KS, Hodges JR (2008a) Names of objects, faces and buildings in mild cognitive impairment. Cortex 44:746–752
Ahmed S, Mitchell J, Arnold R, Nestor PJ, Hodges JR (2008b) Predicting rapid clinical progression in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord 25:170–177
Akram A, Christoffel D, Rocher AB, Bouras C, Kövari E, Perl DP, Morrison JH, Herrmann FR, Haroutunian V, Giannakopoulos P, Hof PR (2008) Stereologic estimates of total spinophilin-immunoreactive spine number in area 9 and the CA1 field: relationship with the progression of Alzheimer’s disease. Neurobiol Aging 29:1296–1307
Akram A, Schmeidler J, Katsel P, Hof PR, Haroutunian V (2010) Increased expression of cholesterol transporter ABCA1 is highly correlated with severity of dementia in AD hippocampus. Brain Res 1318C:167–177
Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovács GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H (2008) Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol 18:484–496
Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM (2002) Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease. Am J Psychiatr 159:738–745
Alladi S, Arnold R, Mitchell J, Nestor PJ, Hodges JR (2006) Mild cognitive impairment: applicability of research criteria in a memory clinic and characterization of cognitive profile. Psychol Med 36:507–515
Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP (2005) Progression of motor impairment and disability in Parkinson disease: a population-based study. Neurology 65:1436–1441
Amlie-Lefond C, Jubelt B (2009) Neurologic manifestations of varicella zoster virus infections. Curr Neurol Neurosci Rep 9:430–434
Archer T, Fredriksson A (2010) Physical exercise attenuates MPTP-induced deficits in mice. Neurotoxicity Res (in press)
Archer T, Beninger RJ, Palomo T, Kostrzewa RM (2010a) Epigenetics and biomarkers in the staging of neuropsychiatric disorders. Neurotoxicity Res (in press)
Archer T, Johansson B, Fredriksson A (2010b) Exercise alleviates parkinsonism: clinical and laboratory evidence. Acta Neurol Scand (in press)
Baillieux H, De Smet HJ, Dobbeleir A, Paquier PF, De Deyn PP, Mariën P (2009) Cognitive and affective disturbances following focal cerebellar damage in adults: a neuropsychological and SPECT study. Cortex, 2009 Oct 1 PMID: 19853848
Bakkour A, Morris JC, Dickerson BC (2009) The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology 72:1048–1055
Barnes J, Whitwell JL, Frost C, Josephs KA, Rossor M, Fox NC (2006) Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer’s disease and frontotemporal lobar degeneration. Arch Neurol 63:1434–1439
Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F (2001) In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage 14:298–309
Basso M, Yang J, Warren L, MacAvoy MG, Varma P, Bronen RA, van Dyck CH (2006) Volumetry of amygdala and hippocampus and memory performance in Alzheimer’s disease. Psychiatr Res 146:251–261
Bastin C, Kerrouche N, Lekeu F, Adam S, Guillaume B, Lemaire C, Aerts J, d’Ydewalle G, Collette F, Salmon E (2010) Controlled memory processes in questionable Alzheimer’s disease: a view from neuroimaging research. J Alzheimers Dis, 2010 Feb 17. PMID: 20164554
Bayulkem K, Lopez G (2010) Nonmotor fluctuations in Parkinson’s disease: clinical spectrum and classification. J Neurol Sci 289:89–92
Beach TG, White CL III, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J, Akiyama H, Adler CH, Arizona Parkinson’s Disease Consortium (2009) Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol 117:169–174
Berchtold NC, Cotman CW (1998) Evolution in the conceptualization of dementia and Alzheimer’s disease: Greco-Roman period to the 1960s. Neurobiol Aging 19:173–189
Berg D, Roggendorf W, Schroder U, Klein R, Tatschner T, Benz P, Tucha O, Preier M, Lange KW, Reiners K, Gerlach M, Becker G (2002) Echogenecity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol 59:999–1005
Bisaglia M, Mammi S, Bubacco L (2009) Structural insights on physiological functions and pathological effects of alpha-synuclein. FASEB J 23:329–340
Bloch A, Probst A, Bissig H, Adams H, Tolnay M (2006) Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 32:284–295
Boeve BF, Lang AE, Litvan I (2003) Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 54(Suppl 5):S15–S19
Boeve BF, Josephs KA, Drubach DA (2008) Current and future management of the corticobasal syndrome and corticobasal degeneration. Handb Clin Neurol 89:533–548
Bohnen NI, Albin RL (2010) The cholinergic system and Parkinson disease. Behav Brain Res, 2010 Jan 7 PMID: 20060022
Bonelli RM, Cummings JL (2007) Frontal-subcortical dementias. Neurologist 14:100–107
Bouras C, Hof PR, Morrison JH (1993) Neurofibrillary tangle dendrites in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathological changes. Neurosci Lett 153:131–135
Bourgeat P, Chételat G, Villemagne VL, Fripp J, Raniga P, Pike K, Acosta O, Szoeke C, Ourselin S, Ames D, Ellis KA, Martins RN, Masters CL, Rowe CC, Salvado O, AIBL Research Group (2010) Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology 74:121–127
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–278
Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18:351–357
Braak H, Braak E (2000) Pathoanatomy of Parkinson’s disease. J Neurol 247:3–10
Braak H, Del Tredici K (2009) Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol 201:1–119
Braak H, Braak E, Yilmazer D, Schultz C, Devos RAI, Jansen ENH (1995) Nigral and extranigral pathology in Parkinson’s disease. J Neural Transm (Suppl): 15–31
Braak H, Braak E, Yilmazer D, de Vos RA, Jansen ENH, Bohl J (1996) Pattern of brain destruction in Parkinson’s and Alzheimer’s disease. J Neural Transm 103:455–490
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003a) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, Rüb U, Gai WP, Del Tredici K (2003b) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110:517–536
Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Braak H, Rüb U, Jansen Steur EN, Del Tredici K, de Vos RA (2005) Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64:1404–1410
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006a) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Braak H, Bohl JR, Müller CM, Rüb U, de Vos RA, Del Tredici K (2006b) Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21:2042–2051
Braak H, Müller CM, Rüb U, Ackermann H, Bratzke H, de Vos RA, Tredici K (2006c) Pathology associated with sporadic Parkinson’s disease—where does it end? J Neural Transm 70:89–97
Braak H, Del Tredici K, Gai WP, Braak E (2009) Alpha-synuclein is not a requisite component of synaptic boutons in the adult human central nervous system. J Chem Neuroanat 20:245–252
Bradley KM, O’Sullivan VT, Soper NDW, Nagy Z, King EMF, Smith AD, Shepstone BJ (2002) Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer’s disease. Brain 125:1772–1781
Brickman AM, Small SA, Fleisher A (2009) Pinpointing synaptic loss caused by Alzheimer’s disease with fMRI. Behav Neurol 21:93–100
Burianova H, McIntosh AR, Grady CL (2010) A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage 49:865–874
Burn DJ (2006) Cortical Lewy body disease and Parkinson’s disease dementia. Curr Opin Neurol 19:572–579
Bush ALH, Allen PA, Kaut KP, Ogrocki PK (2007) Influence of mild cognitive impairment on visual word recognition. Aging Neuropsychol Cogn 14:329–352
Cairns NJ, Ikonomovic MD, Benzinger T, Storandt M, Fagan AM, Shah AR, Reinwald LT, Carter D, Felton A, Holtzman DM, Mintun MA, Klunk WE, Morris JC (2009) Absence of Pittsburgh compound B detection of cerebral amyloid beta in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Arch Neurol 66:1557–1562
Canu E, McLaren DG, Fitzgerald ME, Bendlin BB, Zoccatelli G, Alessandrini F, Pizzini FB, Ricciardi GK, Beltramello A, Johnson SC, Frisoni GB (2010) Microstructural diffusion changes are independent of macrostructural volume loss in moderate to severe Alzheimer’s disease. J Alzheimers Dis 19:963–976
Carlson NE, Moore MM, Dame A, Howieson D, Silbert LC, Quinn JF, Kaye JA (2008) Trajectories of brain loss in aging and the development of cognitive impairment. Neurology 70:828–833
Cedazo-Minguez A, Winblad B (2010) Biomarkers for Alzheimer’s disease and other forms of dementia: clinical needs, limitations and future aspects. Exp Gerontol 45:5–14
Chan D, Janssen JC, Whitwell JL, Watt HC, Jenkins R, Frost C, Rossor MN, Fox NC (2003) Change in rates of cerebral atrophy over time in early-onset Alzheimer’s disease: longitudinal MRI study. Lancet 362:1121–1122
Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC (2002) Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. NeuroReport 13:1939–1943
Chetelat G, Desgranges B, De La Sayette V, Viader F, Berkouk K, Landeau B, Lalevee C, Le Doze F, Dupuy B, Hannequin D, Barn JC, Eustache F (2003a) Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain 126:1955–1967
Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC (2003b) Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology 60:1374–1377
Chetelat G, Landeau B, Eustache F, Mézenge F, Viader F, de la Sayette V, Desgranges B, Baron JC (2005) Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage 27:934–946
Chételat G, Eustache F, Viader F, De La Sayette V, Pélerin A, Mézenge F, Hannequin D, Dupuy B, Baron JC, Desgranges B (2005) FDG-PET measurement is more accurate than neuropsychological assessments to predict global cognitive deterioration in patients with mild cognitive impairment. Neurocase 11:14–25
Chételat G, Fouquet M, Kalpouzos G, Denghien I, De la Sayette V, Viader F, Mézenge F, Landeau B, Baron JC, Eustache F, Desgranges B (2008) Three-dimensional surface mapping of hippocampal atrophy progression from MCI to AD and over normal aging as assessed using voxel-based morphometry. Neuropsychologia 46:1721–1731
Claassen DO, Parisi JE, Giannini C, Boeve BF, Dickson DW, Josephs KA (2008) Frontotemporal dementia mimicking dementia with Lewy bodies. Cogn Behav Neurol 21:157–163
Coleman PD, Flood DG (1987) Neuron numbers and dendritic extent in normal aging and Alzheimer’s disease. Neurobiol Aging 8:521–545
Colosimo C, Hughes AJ, Kilford L, Lees AJ (2003) Lewy body cortical involvement may not always predict dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatr 74:852–856
Cools R, Miyakawa A, Sheridan M, D’Esposito M (2010) Enhanced frontal function in Parkinson’s disease. Brain 133:225–233
Cotman CW, Berchtold NC (2002) Exercise: a behavioural intervention to enhance brain health and plasticity. Trends Neurosci 25:295–301
Coward LA (2010) The hippocampal system as the cortical resource manager: a model connecting psychology, anatomy and physiology. Adv Exp Med Biol 657:315–364
Crews L, Rockenstein E, Masliah E (2010) APP transgenic modeling of Alzheimer’s disease: mechanisms of neurodegeneration and aberrant neurogenesis. Brain Struct Funct 214:111–126
Crivello F, Lemaître H, Dufouil C, Grassiot B, Delcroix N, Tzourio-Mazoyer N, Tzourio C, Mazoyer B (2010) Effects of ApoE-varepsilon4 allele load and age on the rates of grey matter and hippocampal volumes loss in a longitudinal cohort of 1186 healthy elderly persons. Neuroimage, 2010 Jan 6 PMID: 20060049
Cronk BB, Johnson DK, Burns JM; the Alzheimer’s Disease Neuroimaging Initiative (2009) Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Dis Assoc Disord, 2009 Jun 30 PMID: 19571736
Cubo E, Martín PM, Martin-Gonzalez JA, Rodríguez-Blázquez C, Kulisevsky J, ELEP Group Members (2010) Motor laterality asymmetry and nonmotor symptoms in Parkinson’s disease. Mov Disord 25:70–75
De Smet HJ, Baillieux H, Wackenier P, De Praeter M, Engelborghs S, Paquier PF, De Deyn PP, Mariën P (2009) Long-term cognitive deficits following posterior fossa tumor resection: a neuropsychological and functional neuroimaging follow-up study. Neuropsychology 23:694–704
Del Tredisi K, Rub U, De Vos RAI, Bohl JRE, Braak H (2002) Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61:413–426
Delacourt A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, Petit H, Di Menza C (1999) The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 52:1158–1165
Delacourte A (2008) Tau, a biological marker of neurodegenerative diseases. Handb Clin Neurol 89:161–172
DeToledo-Morrell L, Stoub TR, Wilson RS, Bennett DA, Leurgans S, Wuu J, Turner DA (2004) MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging 25:1197–1203
deToledo-Morrell L, Stoub TR, Wang C (2007) Hippocampal atrophy and disconnection in incipient and mild Alzheimer’s disease. Prog Brain Res 163:741–753
Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ (2007) Hippocampal and entorhinal atrophy in mild cognitive atrophy: prediction of Alzheimer disease. Neurology 68:828–836
Dhaenens CM, Schraen-Maschke S, Tran H, Vingtdeux V, Ghanem D, Leroy O, Delplanque J, Vanbrussel E, Delacourte A, Vermersch P, Maurage CA, Gruffat H, Sergeant A, Mahadevan MS, Ishiura S, Buée L, Cooper TA, Caillet-Boudin ML, Charlet-Berguerand N, Sablonnière B, Sergeant N (2008) Overexpression of MBNL1 fetal isoforms and modified splicing of Tau in the DM1 brain: two individual consequences of CUG trinucleotide repeats. Exp Neurol 210:467–478
Di Paola M, Macaluso E, Carlesimo GA, Tomaiuolo F, Worsley KJ, Fadda L, Caltagirone C (2007) Episodic memory impairment in patients with Alzheimer’s disease is correlated with entorhinal cortex atrophy. A voxel-based morphometry study. J Neurol 254:774–781
Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD (1989) Effect of age at onset on progression and mortality in Parkinson’s disease. Neurology 39:1187–1190
Dickerson BC, Eichenbaum H (2010) The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology 35:86–104
Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA (2004) Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol 56:27–35
Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA (2005) Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65:404–411
Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL (2009) The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex 19:497–510
Dickson DW, Uchikado H, Fujishiro H, Tsuboi Y (2010) Evidence in favor of Braak staging of Parkinson’s disease. Mov Disord 25(S1):S78–S82
Duara R, Loewenstein DA, Greig-Custo MT, Raj A, Barker W, Potter E, Schofield E, Small B, Schinka J, Wu Y, Potter H (2010) Diagnosis and staging of mild cognitive impairment, using a modification of the clinical dementia rating scale: the mCDR. Int J Geriatr Psychiatry 25:282–289
Dudas RB, Clague F, Thompson SA, Graham KS, Hodges JR (2005) Episodic and semantic memory in mild cognitive impairment. Neuropsychologia 43:1266–1276
Duong A, Whitehead V, Hanratty K, Chertkow H (2006) The nature of lexico-semantic processing deficits in mild cognitive impairment. Neuropsychologia 44:1928–1935
Dupiereux I, Zorzi W, Quadrio I, Perret-Liaudet A, Kovacs GG, Heinen E, Elmoualij B (2009) Creutzfeldt-jakob, Parkinson, lewy body dementia and Alzheimer diseases: from diagnosis to therapy. Cent Nerv Syst Agents Med Chem 9:2–11
Farina E, Baglio F, Caffarra P, Magnani G, Scarpini E, Appollonio I, Bascelli C, Cheldi A, Nemni R, Franceschi M; Italian Group for Lewy Body Dementia and Dementias Associated to Parkinsonism, Messa G, Mantovani F, Bellotti M, Olivotto F, Alberoni M, Isella V, Regazzoni R, Schiatti E, Vismara C, Falautano M, Barbieri A, Restelli I, Fetoni V, Donato M, Zuffi M, Castiglioni S (2009) Frequency and clinical features of Lewy body dementia in Italian memory clinics. Acta Biomed 80: 57–64
Fayed N, Dávila J, Oliveros A Jr, Medrano J, Castillo J (2010) Correlation of findings in advanced MR techniques with global severity scales in patients with some grade of cognitive impairment. Neurol Res 32:157–165
Fouquet M, Desgranges B, Landeau B, Duchesnay E, Mezenge F, de la Sayette V, Viader F, Baron JC, Eustache F, Chetelat G (2009) Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer’s disease. Brain 132:2058–2067
Fujimi K, Sasaki K, Noda K, Wakisaka Y, Tanizaki Y, Matsui Y, Sekita A, Iida M, Kiyohara Y, Kanba S, Iwaki T (2008) Clinicopathological outline of dementia with Lewy bodies applying the revised criteria: the Hisayama study. Brain Pathol 18:125–317
Fujita S, Kiguchi M, Kobayashi M, Koshikawa N, Waddington JL (2010) Involvement of NMDA receptors in the ventrolateral striatum of rats in apomorphine-induced jaw movements. Brain Res 1322:30–37
Gadre G, Satishchandra P, Mahadevan A, Suja M, Madhusudana S, Sundaram C, Shankar S (2009) Rabies viral encephalitis—clinical determinants in diagnosis with special reference to paralytic form. J Neurol Neurosurg Psychiatry, 2009 Dec 3 PMID: 19965838
Galvin JE, Lee VM, Trojanowski JQ (2001) Synucleinopathies: clinical and pathological implications. Arch Neurol 58:186–190
George JL, Mok S, Moses D, Wilkins S, Bush AI, Cherny RA, Finkelstein DI (2009) Targeting the progression of Parkinson’s disease. Curr Neuropharmacol 7:9–36
Gerlach M, Reiderer P (1999) Time sequences of dopaminergic cell death in Parkinson’s disease: indications for neuroprotective studies. Adv Neurol 80:219–225
Ginanneschi A, Degl’Innocenti F, Magnolfi S, Maurello MT, Catarzi L, Marini P, Amaducci L (1988) Evaluation of Parkinson’s disease: reliability of three rating scales. Neuroepidemiology 7:38–41
Ginanneschi A, Degl’Innocenti F, Maurello MT, Magnolfi S, Marini P, Amaducci L (1991) Evaluation of Parkinson’s disease: a new approach to disability. Neuroepidemiology 10:282–287
Goetz CG (2000) Nineteenth century studies of atypical parkinsonism: Charcot and his Salpetriére school. Adv Neurol 82:1–8
Goetz CG (2006) What’s new? Clinical progression and staging of Parkinson’s disease. J Neural Transm 70:305–308
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L, Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease (2004) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19:1020–1028
Gold BT, Johnsrude IS, Jicha GA, Smith CD (2010) Functional response in ventral temporal cortex differentiates mild cognitive impairment from normal aging. Hum Brain Mapp, 2010 Jan 8 PMID: 20063353
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001a) A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14:21–36
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001b) Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 14:685–700
Grinberg LT, Rüb U, Ferretti RE, Nitrini R, Farfel JM, Polichiso L, Gierga K, Jacob-Filho W, Heinsen H, Brazilian Brain Bank Study Group (2009) The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol 35:406–416
Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, Lipton RB (1999) Memory and mental status correlates of modified Braak staging. Neurobiol Aging 20:573–579
Gunn DG, Naismith SL, Lewis SJ (2010) Sleep disturbances in Parkinson’s disease and their potential role in heterogeneity. J Geriatr Psychiatr Neurol, 2010 Jan 25 PMID: 20101072
Halliday GM, Del Tredici K, Braak H (2006) Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. J Neural Transm (Suppl 70): 99–103
Harding AJ, Halliday GM (2001) Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol 102:355–363
Haroutunian V, Katsel P, Schmeidler J (2009) Transcriptional vulnerability of brain regions in Alzheimer’s disease and dementia. Neurobiol Aging 30:561–573
Hassabis D, Kumaran D, Vann SD, Maguire EA (2007) Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA 104:1726–1731
Hassan A, Whitwell JL, Boeve BF, Jack CR Jr, Parisi JE, Dickson DW, Josephs KA (2010) Symmetric corticobasal degeneration (S-CBD). Parkinsonism Relat Disord 16:208–214
Hauser RA, Pahwa R, Lyons KE, McClain T (2009) Parkinson disease. Available at http://emedicine.medscape.com/article/1151267-overview
Hayes MW, Fung VS, Kimber TE, O’Sullivan JD (2010) Current concepts in the management of Parkinson disease. Med J Aust 192:144–149
Hébert SS, Horré K, Nicolaï L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B (2008) Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci USA 105:6415–6420
Hébert SS, Horré K, Nicolaï L, Bergmans B, Papadopoulou AS, Delacourte A, De Strooper B (2009) MicroRNA regulation of Alzheimer’s amyloid precursor protein expression. Neurobiol Dis 33:422–428
Hoehn MM (1992) The natural history of Parkinson’s disease in the pre-levodopa and post-levodopa eras. Neurol Clin 10:331–339
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Hoehn MM, Yahr MD (1998) Parkinsonism: onset, progression, and mortality. 1967. Neurology 50:318–344
Hoehn MM, Yahr MD (2001) Parkinsonism: onset, progression, and mortality. 1967. Neurology 57(10 Suppl 3): S11–S26
Holman BL, Johnson KA, Gerada B, Carvalho PA, Satlin A (1992) The scintigraphic appearance of Alzheimer’s disease: a prospective study using tecnetium-99 m-HMPAO SPECT. J Nucl Med 33:181–185
Holmes C, Cotterell D (2009) Role of infection in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs 23:993–1002
Hoque KE, Indorkar RP, Sammut S, West AR (2010) Impact of dopamine-glutamate interactions on striatal neuronal nitric oxide synthase activity. Psychopharmacology 207:571–581
Hornykiewicz O (1998) Biochemical aspects of Parkinson’s disease. Neurology 51:S2–S9
Hu WT, Rippon GW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Josephs KA (2009) Alzheimer’s disease and corticobasal degeneration presenting as corticobasal syndrome. Mov Disord 24:1375–1379
Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS (2007) Antidepressant actions of the exercise-regulated gene VGF. Nat Med 13:1476–1782
Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, Clark CM, Glosser G, Stern MB, Gollomp SM, Arnold SE (2000) Alpha-synuclein cortical lewy bodies correlate with dementia in Parkinson’s disease. Neurology 54:1916–1921
Hurwitz A (1989) The benefit of a home exercise regime for ambulatory Parkinson’s disease patients. J Neurosci Nurs 21:180–184
Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225:1168–1170
Ince PG, Perry EK, Morris CM (1998) Dementia with Lewy bodies. A distinct non-Alzheimer dementia syndrome? Brain Pathol 8:299–324
Ishizawa T, Mattlia P, Davies P, Wang D, Dickson DW (2003) Colocalization of tau and α-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 62:389–397
Iwakiri M, Mizukami K, Ikonomovic MD, Ishikawa M, Hidaka S, Abrahamson EE, DeKosky ST, Asada T (2005) Changes in hippocampal GABABR1 subunit expression in Alzheimer’s patients: association with Braak staging. Acta Neuropathol 109:467–474
Iwanaga K, Wakabayashi K, Yoshimoto M, Tomita I, Satoh H, Takashima H, Satoh A, Seto M, Tsujihata M, Tahahashi H (1999) Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology 52:1269–1271
Jack CR Jr, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E (1997) Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology 49:786–794
Jack CR Jr, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC (2004) Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62:591–600
Jack CR Jr, Weigand SD, Shiung MM, Przybelski SA, O’Brien PC, Gunter JL, Knopman DS, Boeve BF, Smith GE, Petersen RC (2008) Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology 70:1740–1752
Jack CR Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC, Alzheimer’s Disease Neuroimaging Initiative (2009) Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain 132:1355–1365
Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9:119–128
Jagust W, Thisted R, Devous MD, Van Heertum R, Mayberg H, Jobst K, Smith AD, Borys N (2001) SPECT perfusion imaging in the diagnosis of Alzheimer’s disease: a clinical-pathologic study. Neurology 56:950–956
Jauhiainen AM, Pihlajamaki M, Tervo S, Niskanen E, Tanila H, Hanninen T, Vanninen RL, Soininen H (2009) Discriminating accuracy of medial temporal lobe volumetry and fMRI in mild cognitive impairment. Hippocampus 19:166–175
Jelic V, Palmer K, Winblad B (2003) Mild cognitive impairment: unanswered questions and future directions. Acta Neurol Scand Suppl 179:100–101
Jellinger KA (2003) α-Synuclein pathology in Parkinson and Alzheimer disease brain: incidence and topographic distribution—a pilot study. Acta Neuropathol 106:191–201
Jellinger KA (2004) Lewy body-related α-synucleinopathy in the aged human brain. J Neural Transm 111:1219–1235
Jellinger KA (2008) A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116:1–16
Jellinger KA (2009a) Formation and development of Lewy pathology: a critical update. J Neurol 256(Suppl 3):S270–S279
Jellinger KA (2009b) A critical evaluation of current staging of α-synuclein pathology in Lewy body disorders. Biochim Biophys Acta 1792:730–740
Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Schild HH, Scheef L (2006) Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging 27:1751–1756
Jessen F, Gür O, Block W, Ende G, Frölich L, Hammen T, Wiltfang J, Kucinski T, Jahn H, Heun R, Maier W, Kölsch H, Kornhuber J, Träber F (2009) A multicenter (1)H-MRS study of the medial temporal lobe in AD and MCI. Neurology 72:1735–1740
Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, Tangalos EG, Boeve BF, Knopman DS, Braak H, Petersen RC (2006) Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol 63:674–681
Jicha GA, Abner E, Schmitt FA, Cooper GE, Stiles N, Hamon R, Carr S, Smith CD, Markesberg WR (2008) Clinical features of mild cognitive impairment differ in the research and tertiary settings. Dement Geriatr Cogn Disord 26:187–192
Jobst KA, Smith AD, Barker CS, Wear A, King EM, Smith A, Anslow PA, Molyneux AJ, Shepstone BJ, Soper N (1992) Association of atrophy of medial temporal lobe with reduced blood flow in the posterior parietotemporal cortex in patients with a clinical and pathological diagnosis of Alzheimer’s disease. J Neurol Neurosurg Psychiatr 55:190–194
Johns EK, Phillips NA, Belleville S, Goupil D, Babins L, Kelner N, Ska B, Gilbert B, Inglis G, Panisset M, de Boysson C, Chertkow H (2009) Executive functions in frontotemporal dementia and Lewy body dementia. Neuropsychology 23:765–777
Josephs KA, Holton JL, Rossor MN, Braendgaard H, Ozawa T, Fox NC, Petersen RC, Pearl GS, Ganguly M, Rosa P, Laursen H, Parisi JE, Waldemar G, Quinn NP, Dickson DW, Revesz T (2003) Neurofilament inclusion body disease: a new proteinopathy? Brain 126:2291–2303
Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, Parisi JE, Dickson DW (2006) Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 66:41–48
Josephs KA, Ahlskog JE, Parisi JE, Boeve BF, Crum BA, Giannini C, Petersen RC (2009) Rapidly progressive neurodegenerative dementias. Arch Neurol 66:201–207
Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK (2008) Controversies over the staging of α-synuclein pathology in Parkinson’s disease. Acta Neuropathol 116:125–128
Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK (2009a) Parkinson disease: extranigral, multisystem, and {alpha}-synuclein “plus”. Arch Neurol 66:914–915
Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK (2009b) Evidence against a reliable staging system of alpha-synuclein pathology in Parkinson’s disease. Neuropathol Appl Neurobiol 35:125–126
Kalpouzos G, Chetelat G, Landeau B, Clochon P, Viader F, Eustache F, Desgranges B (2009) Structural and metabolic correlates of episodic memory in relation to the depth of encoding in normal aging. J Cogn Neurosci 21:372–389
Kanazawa T, Uchihara T, Takahashi A, Nakamura A, Orimo S, Mizusawa H (2008) Three-layered structure shared between Lewy bodies and lewy neurites-three-dimensional reconstruction of triple-labeled sections. Brain Pathol 18:415–422
Kao AW, Racine CA, Quitania LC, Kramer JH, Christine CW, Miller BL (2009) Cognitive and neuropsychiatric profile of the synnucleinopathies: Parkinson disease, dementia with lewy bodies, and multiple system atrophy. Alzheimer Dis Assoc Disord 23:365–370
Karas GB, Scheltens P, Rombouts SA, Visser PJ, van Schijndel RA, Fox NC, Barkhof F (2004) Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage 23:708–716
Katuse O, Iseki E, Marui W, Kosaka K (2003) Developmental stages of cortical Lewy bodies and their relation to axonal transport blockage in brains of patients with Dementia with Lewy bodies. J Neurosci 211:29–35
Kawahara K, Hashimoto M, Bar-On P, Ho GJ, Crews L, Mizuno H, Rockenstein E, Imam SZ, Masliah E (2008) alpha-Synuclein aggregates interfere with Parkin solubility and distribution: role in the pathogenesis of Parkinson disease. J Biol Chem 283:6979–6987
Kawanabe T, Yoritaka A, Shimura H, Oizumi H, Tanaka S, Hattori N (2010) Successful treatment with Yokukansan for behavioral and psychological symptoms of Parkinsonian dementia. Prog Neuropsychopharmacol Biol Psychiatr 34:284–287
Kemper TL (1994) Neuroanatomical and neuropathological changes during aging and in dementia. In: Albert ML, Knoepfel EJE (eds) Clinical neurology of aging, 2nd edn. Oxford University Press, New York, pp 3–67
Kostrzewa RM, Kostrzewa JP, Brus R (2003) Dopamine receptor supersensitivity: an outcome and index of neurotoxicity. Neurotox Res 5:111–118
Kostrzewa RM, Kostrzewa JP, Brown RW, Nowak P, Brus R (2008) Dopamine receptor sensitivity: development, mechanisms, presentation and clinical applicability. Neurotox Res 14:121–128
Kostrzewa RM, Kostrzewa JP, Kostrzewa RA, Brus R, Nowak P (2010) Stereotypic progressions in psychotic behavior. Neurotoxicity Res (in press)
Kövari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, Giannakopoulos P (2003) Lewy body densities in the entorhinal and anterior cingulated cortex predict cognitive deficits in Parkinson’s disease. Acta Neuropathol 106:83–88
Kövari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, Giannakopoulos P (2007) Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology 68:927–931
Kramer ML, Schulz-Schaeffer WJ (2007) Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci 27:1405–1410
Kwak YD, Brannen CL, Qu T, Kim HM, Dong X, Soba P, Majumdar A, Kaplan A, Beyreuther K, Sugaya K (2006a) Amyloid precursor protein regulates differentiation of human neural stem cells. Stem Cells Dev 15:381–389
Kwak YD, Choumkina E, Sugaya K (2006b) Amyloid precursor protein is involved in staurosporine induced glial differentiation of neural progenitor cells. Biochem Biophys Res Commun 344:431–437
Kwak YD, Dantuma E, Merchant S, Bushnev S, Sugaya K (2010) Amyloid-β precursor protein induces glial differentiation of neural progenitor cells by activation of the IL-6/gp130 signaling pathway. Neurotoxicity Res, PMID: 20309664
Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ; the Alzheimer’s Disease Neuroimaging Initiative (2009) Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging, 2009 Aug 4
Lang AE (2007) The progression of Parkinson disease: a hypothesis. Neurology 68:948–952
Lang AE, Boeve BF, Bergeron C (2006) Corticobasal degeneration. In: Jankovic JJ, Tolosa E (eds) Parkinson’s disease and movement disorders. Lippincott and Williams, Philadelphia, pp 186–202
Leverenz JB, Hamilton R, Tsuang DW, Schantz A, Vavrek D, Larson EB, Kukull WA, Lopez O, Galasko D, Masliah E, Kaye J, Woltjer R, Clark C, Trojanowski JQ, Montine TJ (2008) Empiric refinement of the pathologic assessment of lewy-related pathology in the dementia patient. Brain Pathol 18:220–224
Leyhe T, Müller S, Milian M, Eschweiler GW, Saur R (2009) Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 47:2464–2469
Lin DD, Kleinman JT, Wityk RJ, Gottesman RF, Hillis AE, Lee AW, Barker PB (2009) Crossed cerebellar diaschisis in acute stroke detected by dynamic susceptibility contrast MR perfusion imaging. AJNR Am J Neuroradiol 30:710–715
Loewenstein DA, Acevedo A, Potter E, Schinka JA, Raj A, Greig MT, Agron J, Barker WW, Wu Y, Small B, Schofield E, Duara R (2009) Severity of medial temporal atrophy and amnestic mild cognitive impairment: selecting type and number of memory tests. Am J Geriatr Psychiatry 17:1050–1058
Lonie JA, Herrmann L, Donaghey CL, Ebmeier K (2008) Clinical referral patterns and cognitive profile in mild cognitive impairment. Br J Psychiatr 192:59–64
Lonie JA, Herrmann L, Tierney K, Donaghey CL, O’Carroll R, Ebmeier K (2009) Lexical and semantic fluency discrepancy scores in aMCI and early Alzheimer’s disease. J Neuropsychol 3:79–92
Lonie JA, Kalu UG, Sexton CE, Mackay CE, Bastin M, Terriere E, O’Carroll RE, Ebmeier KP (2010) Mild cognitive impairment—a five year follow-up and imaging study. Neurotoxicity Res
Lou JS, Kearns G, Oken B, Sexton G, Nutt J (2001) Exacerbated physical fatigue and mental fatigue in Parkinson’s disease. Mov Disord 16:190–196
Luders E, Gaser C, Jancke L, Schlaug G (2004) A voxel-based approach to gray matter asymmetries. Neuroimage 22:656–664
Lüders E, Steinmetz H, Jäncke L (2002) Brain size and grey matter volume in the healthy human brain. Neuroreport 13:2371–2374
MacDonald AB (2007a) Alzheimer’s disease Braak Stage progressions: reexamined and redefined as Borrelia infection transmission through neural circuits. Med Hypotheses 68:1059–1064
MacDonald AB (2007b) Alzheimer’s neuroborreliosis with trans-synaptic spread of infection and neurofibrillary tangles derived from intraneuronal spirochetes. Curr Microbiol 52:330–332
MacDonald AB, Miranda JM (1987) Concurrent neocortical borreliosis and Alzheimer’s disease. Hum Pathol 18:759–761
Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD (2000) Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 97:4398–4403
Maguire EA, Nannery R, Spiers HJ (2006a) Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain 129:2894–2907
Maguire EA, Woollett K, Spiers HJ (2006b) London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus 16:1091–1101
Marek K, Innis R, van Dyck C, Fussell B, Early M, Eberly S, Oakes D, Seibyl J (2001) [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology 57:2089–2094
Markesbery WR (2010) Neuropathologic alterations in mild cognitive impairment: a review. J Alzheimers Dis 19:221–228
Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR (2006) Neuropathologic substrate of mild cognitive impairment. Arch Neurol 63:38–46
Markesbery WR, Jicha GA, Liu H, Schmitt FA (2009) Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol 68:816–822
Martin WR (2009) Quantitative estimation of regional brain iron with magnetic resonance imaging. Parkinsonism Relat Disord 15(Suppl 3):S215–S218
Martorana A, Mori F, Esposito Z, Kusayanagi H, Monteleone F, Codecà C, Sancesario G, Bernardi G, Koch G (2009) Dopamine modulates cholinergic cortical excitability in Alzheimer’s disease patients. Neuropsychopharmacology 34:2323–2328
Matsuda H, Kitayama N, Ohnishi T, Asada T, Nakano S, Sakamoto S, Imabayashi E, Katoh A (2002) Longitudinal evaluation of both morphologic and functional changes in the same individuals with Alzheimer’s disease. J Nucl Med 43:304–311
Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M (2000) Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol 100:285–290
Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mulugeta E, Rosén E, Aarsland D, Visser PJ, Schröder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Pirttilä T, Wallin A, Jönhagen ME, Minthon L, Winblad B, Blennow K (2009) CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302:385–393
Meguro K, LeMestric C, Landeau B, Desgranges B, Eustache F, Baron JC (2001) Relations between hypometabolism in the posterior association neocortex and hippocampal atrophy in Alzheimer’s disease: a PET/MRI correlative study. J Neurol Neurosurg Psychiatr 71:315–321
Merims D, Freedman M (2008) Cognitive and behavioural impairment in Parkinson’s disease. Int Rev Psychiatry 20:364–373
Mevel K, Desgranges B, Baron JC, Landeau B, De la Sayette V, Viader F, Eustache F, Chetelat G (2007) Detecting hippocampal hypometabolism in mild cognitive impairment using automatic voxel-based approaches. NeuroImage 37:18–25
Miklossy J, Khalili K, Gern L, Ericson RL, Darekar P, Bolle L, Hurlimann J, Paster BJ (2004) Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease. J Alzheimers Dis 6:639–649
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE (1997) Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 42:85–94
Mizuno K, Wakai M, Takeda A, Sobue G (2000) Medial temporal atrophy and memory impairment in early stage of Alzheimer’s disease: an MRI volumetric and memory assessment study. J Neurol Sci 173:18–24
Mizusawa H (1997) Corticobasal degeneration. Rinsho Shinkeigaku 37:1131–1133
Moncrieff J, Leo J (2010) A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med 20:1–14
Mori E, Yoneda Y, Yamashita H, Hirono N, Ikeda M, Yamadori A (1997) Medial temporal structures relate to memory impairment in Alzheimer’s disease: an MRI volumetric study. J Neurol Neurosurg Psychiatr 63:214–221
Mori F, Tanji K, Zhang H, Kakita A, Takahashi H, Wakabayashi K (2008) Alpha-synuclein pathology in the neostriatum in Parkinson’s disease. Acta Neuropathol 115:453–459
Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L (2001) Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol 58:397–405
Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA (2009) Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol 66:1469–1475
Mosconi L (2005) Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease: FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging 32:486–510
Mukaetova-Ladinska EB, Xuereb JH, Garcia-Sierra F, Hurt J, Gertz HJ, Hills R, Brayne C, Huppert FA, Paykel ES, McGee MA, Jakes R, Honer WG, Harrington CR, Wischik CM (2009) Lewy body variant of Alzheimer’s disease: selective neocortical loss of t-SNARE proteins and loss of MAP2 and alpha-synuclein in medial temporal lobe. ScientificWorld J 9:1463–1475
Müller CM, de Vos RA, Maurage CA, Thal DR, Tolnay M, Braak H (2005) Staging of sporadic Parkinson disease-related α-synuclein pathology: inter- and intra-rater reliability. J Neuropathol Exp Neurol 64:623–628
Nagy Z, Hindley NJ, Braak H, Braak E, Yilmazer-Hanke DM, Schultz C, Barnetson L, King EM, Jobst KA, Smith AD (1999a) The progression of Alzheimer’s disease from limbic regions to the neocortex: clinical, radiological and pathological relationships. Dement Geriatr Cogn Disord 10:115–120
Nagy Z, Hindley NJ, Braak H, Braak E, Yilmazer-Hanke DM, Schultz C, Barnetson L, Jobst KA, Smith AD (1999b) Relationship between clinical and radiological diagnostic criteria for Alzheimer’s disease and the extent of neuropathology as reflected by ‘stages’: a prospective study. Dement Geriatr Cogn Disord 10:109–114
Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR (2007) Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 66:1136–1146
Nelson PT, Braak H, Markesbery WR (2009) Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol 68:1–14
Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, Xu LO, Smith CD, Markesbery WR (2010) Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol 257:359–366
Nestor PJ, Scheltens P, Hodges JR (2004) Advances in the early detection of Alzheimer’s disease. Nat Med 10(Supl): 534–541
Nordberg A, Rinne JO, Kadir A, Långström B (2010) The use of PET in Alzheimer disease. Nat Rev Neurol 6:78–87
Nowak P, Nitka D, Kwieciński A, Jośko J, Drab J, Pojda-Wilczek D, Kasperski J, Kostrzewa RM, Brus R (2009) Neonatal co-lesion by DSP-4 and 5, 7-DHT produces adulthood behavioral sensitization to dopamine D(2) receptor agonists. Pharmacol Rep 61:311–318
Nowak P, Kostrzewa RA, Skaba D, Kostrzewa RM (2010) Acute L: -DOPA effect on hydroxyl radical- and DOPAC-levels in striatal microdialysates of parkinsonian rats. Neurotox Res 17:299–304
O’Keeffe GC, Mitchell AW, Barker RA (2009) Biomerkers in Huntington’s and Parkinson’s disease. Ann NY Acad Sci 1180:97–110
Oguru M, Tachibana H, Toda K, Okuda B, Oka N (2009) Apathy and depression in Parkinson disease. J Geriatr Psychiatry Neurol 23:35–41
Ohnishi T, Matsuda H, Tabira T, Asada T, Uno M (2001) Changes in brain morphology in Alzheimer disease and normal aging: is Alzheimer disease an exaggerated aging process. AJNR Am J Neuroradiol 22:1680–1685
Okazaki K, Fu YJ, Nishihira Y, Endo M, Fukushima T, Ikeuchi T, Okamoto K, Onodera O, Nishizawa M, Takahashi H (2009) Alzheimer’s disease: report of two autopsy cases with a clinical diagnosis of corticobasal degeneration. Neuropathology, 2009 Sep 22 PMID: 19780981
Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, Thal LJ (1998) Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology 51:351–357
Palmer K, Berger AK, Monastero R, Winblad B, Bäckman L, Fratiglioni L (2007) Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology 68:1596–1602
Palmer K, Bäckman L, Winblad B, Fratiglioni L (2008a) Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry 16:603–611
Palmer K, Bäckman L, Winblad B, Fratiglioni L (2008b) Early symptoms and signs of cognitive deficits might not always be detectable in persons who develop Alzheimer’s disease. Int Psychogeriatr 20:252–258
Parker-Pope T (2001) For a healthy brain you really need to use your head—physical and mental exercise can stave off mental decline. Wall St J Eur, November 26, 2001, 8
Parkkinen L, Soininen H, Laakso M, Alafuzoff I (2001) Alpha-synuclein pathology is highly dependent on the case selection. Neuropathol Appl Neurobiol 27:314–325
Parkkinen L, Soininen H, Alafuzoff I (2003) Regional distribution of alpha-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol 62:363–367
Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I (2005) Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol 57:82–91
Parkkinen L, Pirttila T, Alafuzoff I (2008) Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 115:399–407
Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP (1985) Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA 82:4531–4534
Pennanen C, Testa C, Laakso MP, Hallikainen M, Helkala EL, Hänninen T, Kivipelto M, Könönen M, Nissinen A, Tervo S, Vanhanen M, Vanninen R, Frisoni GB, Soininen H (2005) A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry 76:11–14
Pennanen C, Testa C, Boccardi M, Laakso MP, Hallikainen M, Helkala EL, Hänninen T, Kivipelto M, Könönen M, Nissinen A, Tervo S, Vanhanen M, Vanninen R, Frisoni GB, Soininen H (2006) The effect of apolipoprotein polymorphism on brain in mild cognitive impairment: a voxel-based morphometric study. Dement Geriatr Cogn Disord 22:60–66
Pereira AC, Wu W, Small SA (2007) Imaging-guided microarray: isolating molecular profiles that dissociate Alzheimer’s disease from normal aging. Ann N Y Acad Sci 1097:225–238
Petersen RC, Morris JC (2005) Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 62:1160–1163
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308
Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG, Braak H, Kokmen E (2006) Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 63:665–672
Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR Jr, Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, Weiner MW (2010) Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 74:201–209
Petracca GM, Jorge RE, Acion L, Weintraub D, Robinson RG (2009) Frequency and correlates of involuntary emotional expression disorder in Parkinson’s disease. J Neuropsychiatr Clin Neurosci 21:406–412
Pievani M, Agosta F, Pagani E, Canu E, Sala S, Absinta M, Geroldi C, Ganzola R, Frisoni GB, Filippi M (2010) Assessment of white matter tract damage in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp, 2010 Feb 16 PMID: 20162601
Pihlajamäki M, O’keefe K, Bertram L, Tanzi RE, Dickerson BC, Blacker D, Albert MS, Sperling RA (2010) Evidence of Altered Posteromedial Cortical fMRI Activity in Subjects at Risk for Alzheimer Disease. Alzheimer Dis Assoc Disord 24:28–36
Prabhu S, Sripathi H, Gupta S, Prabhu M (2009) Childhood herpes zoster: a clustering of ten cases. Indian J Dermatol 54:62–64
Price JL (1993) The relationship between tangle and plaque formation during healthy aging and mild dementia. Neurobiol Aging 14:661–663
Przuntek H (1992) Early diagnosis in Parkinson’s disease. J Neural Transm 38(Suppl):105–114
Przuntek H, Müller T (eds) (1999) Diagnosis and treatment in Parkinson’s disease—state of the art. Springer, Wien, New York
Przuntek H, Müller T, Reiderer P (2004) Diagnostic staging of Parkinson’s disease: conceptual aspects. J Neural Transm 111:201–216
Randall AD, Witton J, Booth C, Hynes-Allen A, Brown JT (2010) The functional neurophysiology of the amyloid precursor protein (APP) processing pathway. Neuropharmacology, 2010 Feb 15 PMID: 20167227
Rasgon NL, Kenna HA, Wroolie TE, Kelley R, Silverman D, Brooks J, Williams KE, Powers BN, Hallmayer J, Reiss A (2009) Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol Aging, 2009 Dec 24 PMID: 20031276
Raz N (1996) Neuroanatomy of aging brain: evidence from structural MRI. In: Bigler ED (ed) Neuroimaging II: clinical application. Plenum Press, New York, pp 273–300
Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD (1997) Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal grey matter. Cereb Cortex 7:268–282
Rebeiz JJ, Kolodny EH, Richardson EP Jr (1968) Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 18:20–33
Reiderer P, Foley P (2002) Mini-review: multiple developmental forms of parkinsonism. The basis for further research as to the pathogenesis of parkinsonism. J Neural Transm 109:1469–1475
Reitz C, Brickman AM, Brown TR, Manly J, DeCarli C, Small SA, Mayeux R (2009) Linking hippocampal structure and function to memory performance in an aging population. Arch Neurol 66:1385–1392
Remy F, Mirrashed F, Campbell B, Richter W (2005) Verbal episodic memory impairment in Alzheimer’s disease: a combined structural and functional MRI study. NeuroImage 25:253–266
Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, Dannals RF, Mathis CA, Klunk WE, Ferrucci L, Kraut MA, Wong DF (2010) Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology 24:807–815
Riekkinen P, Rinne UK, Pelliniemi TT, Sonninen V (1975) Interaction between dopamine and phospholipids. Studies of the substantia nigra in Parkinson disease patients. Arch Neurol 32:25–27
Rosen AC, Prull MW, Gabrieli JD, Stoub T, O’Hara R, Friedman L, Yesavage JA, de Toledo-Morrell L (2003) Differential associations between entorhinal and hippocampal volumes and memory performance in older adults. Behav Neurosci 117:1150–1160
Rosen AC, Gabrieli JD, Stoub T, Prull MW, O’Hara R, Yesavage J, de Toledo-Morrell L (2005) Relating medial temporal lobe volume to frontal fMRI activation for memory encoding in older adults. Cortex 41:595–602
Sánchez-Benavides G, Gómez-Ansón B, Molinuevo JL, Blesa R, Monte GC, Buschke H, Peña-Casanova J (2009) Medial temporal lobe correlates of memory screening measures in normal ageing, MCI, and AD. J Geriatr Psychiatry Neurol, 2009 Dec 30 PMID: 20029056
Schmahmann JD, Pandya DN (2006) Fiber pathways of the brain. Oxford University Press, New York
Schnaudigel S, Preul C, Ugur T, Mentzel HJ, Witte OW, Tittgemeyer M, Hagemann G (2010) Positional brain deformation visualized with magnetic resonance morphometry. Neurosurgery 66:376–384
Schnell MJ, McGettigan JP, Wirblich C, Papaneri A (2010) The cell biology of rabies virus: using stealth to reach the brain. Nat Rev Microbiol 8:51–61
Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ (2010) The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol 67:64–70
Simic G, Stanic G, Mladinov M, Jovanov-Milosevic N, Kostovic I, Hof PR (2009) Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol 35:532–554
Sironi F, Trotta L, Antonini A, Zini M, Ciccone R, Della Mina E, Meucci N, Sacilotto G, Primignani P, Brambilla T, Coviello DA, Pezzoli G, Goldwurm S (2010) alpha-Synuclein multiplication analysis in Italian familial Parkinson disease. Parkinsonism Relat Disord 16:228–231
Small SA (2005) Alzheimer disease, in living color. Nat Neurosci 8:404–405
Smith CD (2007) Mild cognition impairment is too late: the case for presymptomatic detection and treatment for Alzheimer’s disease. Cogn Sci 3:127–177
Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison JM (1999) Altered brain activation in cognitively intact individuals at high risk for Alzheimer’s disease. Neurology 53:1391–1396
Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, Markesbery WR (2007) Brain structural alterations before mild cognitive impairment. Neurology 68:1268–1273
Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ (2007) Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 62:406–413
Sonnen JA, Montine KS, Quinn JF, Kaye JA, Breitner JC, Montine TJ (2008) Biomarkers for cognitive impairment and dementia in elderly people. Lancet Neurol 7:704–714
Sonnen JA, Montine KS, Quinn JF, Breitner JC, Montine TJ (2010) Cerebrospinal fluid biomarkers in mild cognitive impairment and dementia. J Alzheimers Dis 19:301–309
Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, Laviolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA (2010) Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med 12:27–43
Spiers HJ, Maguire EA (2006) Spontaneous mentalizing during an interactive real world task: an fMRI study. Neuropsychologia 44:1674–1682
Squires L, Zola-Morgan S (1991) The medial temporal lobe memory system. Science 253:1380–1386
Stebbins GT, Murphy CM (2009) Diffusion tensor imaging in Alzheimer’s disease and mild cognitive impairment. Behav Neurol 21:39–49
Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, Detoledo-Morrell L (2008) Rate of entorhinal and hippocampal atrophy in incipient and mild AD: Relation to memory function. Neurobiol Aging, 2008 Sep 20
Streit WJ, Braak H, Xue QS, Bechmann I (2009) Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol 118:475–485
Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, Suliman H, Wagner M, Schild HH, Jessen F (2010) Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement Geriatr Cogn Disord 29:75–81
Sugaya K (2008) Mechanism of glial differentiation of neural progenitor cells by amyloid precursor protein. Neurodegener Dis 5:170–172
Sugaya K, Alvarez A, Marutle A, Kwak YD, Choumkina E (2006) Stem cell strategies for Alzheimer’s disease therapy. Panminerva Med 48:87–96
Sugaya K, Kwak YD, Ohmitsu O, Marutle A, Greig NH, Choumrina E (2007) Practical issues in stem cell therapy for Alzheimer’s disease. Curr Alzheimer Res 4:370–377
Sulzer D (2007) Multiple hit hypotheses for dopamine neuron loss in parkinson’s disease. Trends Neurosci 30:244–250
Tabert MH, Liu X, Doty RL, Serby M, Zamora D, Pelton GH, Marder K, Albers MW, Stern Y, Devanand DP (2005) A 10-item smell identification scale related to risk for Alzheimer’s disease. Ann Neurol 58:155–160
Tang CC, Poston KL, Dhawan V, Eidelberg D (2010) Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson’s disease. J Neurosci 30:1049–1056
Teipel SJ, Stahl R, Dietrich O, Schoenberg SO, Perneczky R, Bokde AL, Reiser MF, Moller HJ, Hampel H (2007) Multivariate network analysis of fiber tract integrity in Alzheimer’s disease. NeuroImage 34:985–995
Terriere E, Sharman M, Donaghey C, Herrmann L, Lonie J, Strachan M, Dougall N, Best J, Ebmeier K, Pimlott S, Patterson J, Wyper D (2008) Alpha-4-beta-2-nicotinic receptor binding with 5-1A in Alzheimer’s disease: methods of scan analysis. Neurochem Res 33:643–651
Terriere E, Dempsey M, Herrmann L, Tierney K, Lonie J, O’Carroll R, Pimlott S, Wyper D, Herholz K, Ebmeier K (2009) 5-(123)I-A-85380 binding to the alpha-4-beta-2-nicotinic receptor in mild cognitive impairment. Neurobiol Aging, PMID: 19036475
Terry RD, Peck A, DeTeresa R (1981) Some morphometric aspects of the brain in senile dementia of Alzheimer’s type. Ann Neurol 10:184–192
Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW (2003) Dynamics of grey matter loss in Alzheimer’s disease. J Neurosci 23:994–1005
Uversky VN (2009) Intrinsic disorder in proteins associated with neurodegenerative diseases. Front Biosci 14:5188–5238
Uversky VN, Eliezer D (2009) Biophysics of Parkinson’s disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci 10:483–499
van Hilten JJ, van der Zwan AD, Zwinderman AH, Roos RA (1994) Rating impairment and disability in Parkinson’s disease: evaluation of the Unified Parkinson’s Disease Rating Scale. Mov Disord 9:84–88
Vemuri P, Whitwell JL, Kantarci K, Josephs KA, Parisi JE, Shiung MS, Knopman DS, Boeve BF, Petersen RC, Dickson DW, Jack CR Jr (2008) Antemortem MRI based STructural Abnormality iNDex (STAND)-scores correlate with postmortem Braak neurofibrillary tangle stage. Neuroimage 42:559–567
Villain N, Desgranges B, Viader F, de la Sayette V, Mezenge F, Landeau B, Baron JC, Eustache F, Chetelat G (2008) Relations between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. J Neurosci 28:6174–6181
Volpato C, Signorini M, Meneghello F, Semenza C (2009) Cognitive and personality features in Parkinson disease: 2 sides of the same coin? Cogn Behav Neurol 22:258–263
Wadia PM, Lang AE (2007) The many faces of corticobasal degeneration. Parkinsonism Rel Disord 13:S336–S340
Wakabayashi K, Takahashi H (1997) Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol 38(Suppl 2):2–7
Wakabayashi K, Mori F, Takahashi H (2006) Progression patterns of neuronal loss and Lewy body pathology in the substantia nigra in Parkinson’s disease. Parkinsonism Relat Disorder 12:92–98
Wakabayashi K, Tanji K, Mori F, Takahashi H (2007) The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology 27:494–506
Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T (2003) Age-associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol 106:374–382
Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu Y, Jiang T, Li K (2006) Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state MRI. NeuroImage 31:496–504
Wang L, Harms MP, Staggs JM, Xiong C, Morris JC, Csernansky JG, Galvin JE (2010) Donepezil treatment and changes in hippocampal structure in very mild Alzheimer disease. Arch Neurol 67:99–106
Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC (2001) Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex 11:868–877
Watson R, Blamire AM, O’Brien JT (2009) Magnetic resonance imaging in Lewy body dementias. Dement Geriatr Cogn Disord 28:493–506
West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal aging and Alzheimer’s disease. Lancet 344:769–772
Whitwell JL (2010) Progression of atrophy in Alzheimer’s disease and related disorder. Neurotoxicity Res (in press), PMID: 20352396
Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR Jr (2007) 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain 130:1777–1786
Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, Weigand SD, Vemuri P, Senjem ML, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW, Jack CR Jr (2008) MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 71:743–749
Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, Senjem ML, Shiung MM, Boeve BF, Knopman DS, Parisi JE, Dickson DW, Petersen RC, Jack CR Jr, Josephs KA (2009) Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain 132:2932–2946
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC (2004) Mild cognitive impairment—beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256:240–246
Wolman L, Roy S (1969) The substantia nigra in Parkinsonism. J Clin Pathol 22:507–508
Wolters EC (2009) Non-motor extranigral signs and symptoms in Parkinson’s disease. Parkinsonism Relat Disord 15(Suppl 3):S6–S12
Wolters EC, Francot C, Bergmans P, Winogrodzka A, Booij J, Berendse HW, Stoof JC (2000) Preclinical (premotor) Parkinson’s disease. J Neurol 247:103–109
Woodard JL, Seidenberg M, Nielson KA, Antuono P, Guidotti L, Durgerian S, Zhang Q, Lancaster M, Hantke N, Butts A, Rao SM (2009) Semantic memory activation in amnestic mild cognitive impairment. Brain 132:2068–2078
Wu W, Small SA (2006) Imaging the earliest stages of Alzheimer’s disease. Curr Alzheimer Res 3:529–539
Wu W, Brickman AM, Luchsinger J, Ferrazzano P, Pichiule P, Yoshita M, Brown T, DeCarli C, Barnes CA, Mayeux R, Vannucci SJ, Small SA (2008) The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann Neurol 64:698–706
Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX (2006) Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology 66:1845–1849
Xuereb JH, Brayne C, Dufouil C, Gertz H, Wischik C, Harrington C, Mukaetova-Ladinska E, McGee MA, O’Sullivan A, O’Connor D, Paykel ES, Huppert FA (2000) Neuropathological findings in the very old. Results from the first 101 brains of a population-based longitudinal study of dementing disorders. Ann N Y Acad Sci 903:490–496
Yamamoto R, Iseki E, Marui W, Togo T, Katsuse O, Kato M, Isojima D, Akatsu H, Kosaka K, Arai H (2005) Non-uniformity in the regional pattern of Lewy pathology in brains of dementia with Lewy bodies. Neuropathology 25:188–194
Yazici KM, Kapucu Ö, Erbas B, Varoglu E, Gülec C, Bekdik CF (1992) Assessment of changes in regional cerebral blood flow in patients with depression using the 99m Tc HMPAO single photon emission tomography method. Eur J Nucl Med 19:1038–1043
Yener GG, Turkuaz Alzheimer Working (TAC) Group (2009) The neuropsychiatric inventory scores change across the mini mental state examination ranges in patients with Alzheimer’s disease: a multicenter study in Turkey. Cogn Behav Neurol 22:264–269
Zaccai J, Ince P, Brayne C (2006) Population-based neuropathological studies of dementia: design, methods and areas of investigation—a systematic review. BMC Neurol 9:2
Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG, MRC Cognitive Function, Ageing Neuropathology Study (2008) Patterns and stages of alpha-synucleinopathy: relevance in a population-based cohort. Neurology 70:1042–1048
Zerboni L, Sobel RA, Ramachandran V, Rajamani J, Ruyechan W, Abendroth A, Arvin A (2010) The expression of varicella-zoster virus immediate early regulatory protein IE63 in neurons of latently infected human sensory ganglia. J Virol 84:3421–3430
Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW (2007) Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology 68:13–19
Zhou XW, Li X, Bjorkdahl C, Sjogren MJ, Alafuzoff I, Soininen H, Grundke-Iqbal I, Iqbal K, Winblad B, Pei JJ (2006) Assessments of the accumulation severities of amyloid beta-protein and hyperphosphorylated tau in the medial temporal cortex of control and Alzheimer’s brains. Neurobiol Dis 22:657–668
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Archer, T., Kostrzewa, R.M., Beninger, R.J. et al. Staging Neurodegenerative Disorders: Structural, Regional, Biomarker, and Functional Progressions. Neurotox Res 19, 211–234 (2011). https://doi.org/10.1007/s12640-010-9190-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-010-9190-2