Abstract
Tick vaccines are important component of integrated pest management for sustainable control of tick and tick born diseases. Immune responses against rHaa86 (homologue of Bm86) recombinant Hyalomma tick antigen were determined in experimental crossbred calves. The humoral antibody responses of the calves were measured against rHaa86 in an optimized ELISA format. The expression of the interferon gamma (IFN-γ), was also evaluated in the culture supernatant of blood culture from blood samples of the experimental calves. The expression patterns were studied after stimulating the blood cells in vitro with rHaa86 antigen and subsequently optical density was measured against IFN-γ. The results were expressed as stimulation indices. All the rHaa86 immunized animal showed strong humoral antibody response just after 1st vaccination and reach to pick after 2nd booster and thereafter maintained up to days 120 from post primary immunization. The humoral antibody response was dominated by IgG1 against IgG2 throughout the period of antibody monitoring. The standard graph of bovine recombinant IFN-γ was plotted which showed a significant difference in SI and OD value up to 200 pg/ml. The lowest detectable value of IFN-γ was 20 pg/ml and SI at this level is 1.16 which is greater than maximum SI calculated from individual calf. The IFN-γ response never reached at significant level and the IgG1 response was dominated over IgG2 response throughout the period of experiment. Since IgG2 and IFN-γ are interlinked, the present study established the Th2 response as a possible mode of mechanism of conferring antibody mediated protection against challenged ticks.
Similar content being viewed by others
Introduction
In India, although the importance of controlling ticks and tick-borne diseases (TTBDs) has been given top priority, the progress on immunological control of the tick has not yet reached to the ultimate level of vaccine development suitable to Indian condition. Earlier, attempts have been made to immunize laboratory animals and cattle against ticks species with crude and purified antigens and significant protection has been achieved (Singh and Ghosh 2003; Ghosh et al. 2005). However, none of the studies have reached to the development of vaccine against the target tick species.
The successful identification and isolation of first novel concealed antigen from tick gut was Bm86 and latter the protein has been expressed in different expression system and commercial vaccines have been developed and marketed (Willadsen et al. 1988, 1989; Cobon and Willadsen 1990; Rand et al. 1989; Rodriguez et al. 1994). When rBm86 protein inoculated into animal, body defense system treated it as foreign body and activation of immune system occur. Following ingestion of the blood from immunized cattle, the antibodies together with other components of the immune system such as complement, cause lysis of the gut epithelial cells of tick, leading either to death or to disruption of normal gut physiology and reduce growth and egg-laying ability (Willadsen et al. 1989; Kemp et al. 1986). Regulation of humoral immune responses is multifactorial involving appropriate activation, co-stimulation and the presence of specific soluble factors (Estes and Brown 2002). In vitro studies have suggested that antibody responses in cattle polarized to type 1 or type 2 responses could be linked to specific IgG subclass expression patterns. Bovine IgG1 expression is positively regulated by IL-4, and IgG2 expression by IFN-γ (Estes et al. 1994, 1995).
Taking the lead from the work of the homologue of Bm86, Haa86 has been cloned and expressed in both prokaryotic and eukaryotic systems. The expressed protein has been characterized and tested against homologous and heterologous challenge infestation (Azhahianambi et al. 2009a, b; Jeyabal et al. 2010; Kumar et al. 2012). However, the immune response to rHaa86 is not well characterized in natural host. With this background the present experiment was undertaken to study the immune response of cattle following immunization with rHaa86 antigen.
Materials and methods
Cattle
Cross-bred (Bos indicus × Bos taurus) bovine calves (n = 9) were procured from Livestock Production and Management section of the Institute, Izatnagar just after weaning and maintained in the tick proof shed of the Division of Parasitology. The calves were fed on a daily ration of concentrate and wheat bhusa with ad lib drinking water. Each calf was supplemented with vitamin A and mineral mixture at regular interval. The experimental animals were maintained according to the guidelines of Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA), a statutory Indian body.
rHaa86
The full length gene of 1.7 kb size of Bm86 orthologue of Hyalomma anatolicum anatolicum (Haa86) cloned in the cloning vector pET 32a and transformed in Escherichia coli BL21(DE3)PLysS strain was available in the Entomology laboratory, Division of Parasitology. The clones were revived by sub-culturing in Luria Bartani (LB) broth supplemented with ampicilline (100 μg/ml) and chloramphanicol (34 μg/ml). For mass scale production of desired protein, freshly grown overnight cultures were inoculated in LB medium (1,000 ml) and incubated at 37 °C with shaking. When the OD reached at 0.5–0.6, the cells were induced with 1 mM isopropyl-b-d-thioglactopyranoside (IPTG) and incubated further with shaking. Bacterial cells were harvested by centrifugation and stored at −20 °C. To purify the expressed protein, the cell pellet was resuspended in lysis buffer (containing urea, Tris–Cl and NaH2PO4) and mixed by vortexing. To enhance the lysis of cells the suspension was stirred for 2 h at 22 °C in the shaking incubator at 220 rpm and sonicated at 10 μm for 5–6 times for 45 s each after 1 min rest. The cell lysate was obtained by centrifugation and stored at −20 °C. The lysate containing the solubilized protein was subjected to purification by nickel-nitrilotriacetic acid (Ni–NTA) agarose resin (Qiagen, Germany). The level of recombinant protein present in fractions collected during elution was confirmed by SDS-PAGE. The fractions were pooled and dialyzed using 7,000 Da cut-off dialysis membrane (Pierce, UK) against decreasing strength of urea and finally in PBS (pH 7.2), is to remove the urea and re-nature/refold the protein. The resultant buffer containing recombinant rHaa86 was subjected to ultra filtration using 50 kDa cut off ultra filter (Pall life sciences). The protein was resolved in SDS-PAGE (12 % gel) along with bovine serum albumin (BSA) in the concentrations of 1–10 μg per 20 μl of buffer. The band thickness of protein sample matching with a particular concentration of BSA was used to calculate the concentration of the rHaa86. The protein sample was labeled, mixed with cocktail of protease inhibitors (Amresco, USA) and stored at −20 °C.
Gel purification of rHaa86
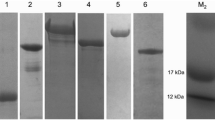
The Ni–NTA purified rHaa86 was eluted from 8 % non-reducing polyacrylamide gels. The gel slices were mixed thoroughly with PBS, pH 7.4 containing cocktail of protease inhibitors and incubated the mixture over night at 4 °C on magnetic stirrer. The targeted protein was collected from the supernatant by centrifugation at 15,000 rpm for 30 min at 4 °C. The eluted protein was checked by SDS-PAGE. The concentration of eluted protein was estimated by Fluorometer (Cubett, Invitrogen, USA) and stored at −20 °C. This gel purified protein was used for in vitro antigenic stimulation of lymphocytes in blood culture.
Immunization
Nine cross breed calves (10–12 month old) were treated with Albendazole [Albomol®] at 7.5 mg/kg body weight orally one month prior to immunization. For immunization, animals were divided randomly into three groups comprising of three animals in each group. Groups 1 immunized with rHaa86 and group 2 and 3 was kept as adjuvant and negative control (inoculated with PBS only), respectively. The frozen rHaa86 protein samples (100 μg/ml) were thawed and emulsified thoroughly with equal volume of adjuvant (10 % Montanide 888 in mineral oil). Animals of group 1 was inoculated with 2 ml of reconstituted rHaa86 vaccine on 0, 30 and 60th day. The immunization was carried out by deep intramuscular inoculation in the glutial muscle.
Collection of blood and serum
To quantify expression of IFN-γ, whole blood samples were collected with anti coagulant (10 IU of heparin per ml of blood) at different interval from pre-immunized and post-immunized calves under sterile condition. Separate blood samples were also collected without anti-coagulant for separation of serum. The collection schedule was day 0 (prior to primary immunization), 21st day, 45th day, 75th day, 100th day and 140th day post immunization. The serum samples were stored at −80 °C for analysis of humoral immune responses.
Whole blood culture and antigenic stimulation of lymphocytes
The whole blood culture was done for antigenic stimulation of lymphocytes as previously described (Weynants et al. 1995; Hasvold et al. 2002). Three, 1 ml aliquots of each blood sample collected from the experimental animals were distributed in 24 wells culture plate (Axygen, USA). For negative control, 100 μl of PBS was added to one aliquot, while for positive control 5 μg concanavaline A in 100 μl was added to second aliquot. For antigenic stimulation, 20 μg of rHaa86 was mixed with third whole blood aliquot. The culture was incubated at 37 °C in a humified atmosphere with 5 % CO2 for 30 h. For gross assessment and validity of test, following incubation, lymphocytes were isolated by centrifugation using Histopaque-1077 (Talwar and Gupta 1992) and viability of cells was assessed by trypan blue. To check the sterility of culture, integrity of blood cells and proliferation of lymphocytes, Giemsa stained blood smears were prepared and examined microscopically. The culture supernatant was stored at −80 °C until assayed for the IFN-γ content.
Measurement of IFN-γ response in culture supernatant
For measurement of the IFN-γ in culture supernatant, Sandwich ELISA was optimized and standard graph was plotted using recombinant bovine IFN-γ. The microtiter plates (Nunc, Denmark) were coated with 100 μl/well of mouse anti-bovine IFN-γ (AbD serotec, UK), by company recommended concentration of 5 μg/ml in coating buffer (0.02 carbonate–bicarbonate buffer, pH 9.6), and incubated overnight at 4 °C. The recombinant IFN-γ (AbD serotec,UK) was diluted with PBS at a concentration of 10, 4, 2, 1, 500, 200, 100, 50 and 20 pg. Simultaneously, culture supernatant was serially diluted as 1:10, 1:50 and 1:100 in diluting solution (washing buffer containing 1 % BSA). Each concentration of IFN-γ was probed with different dilution of culture supernatant at 100 μl level. The biotin conjugate, mouse anti-bovine IFN-γ:biotin (AbD serotec, UK), was diluted to 1, 2, 3 and 4 μg per ml in diluting solution and was used in checkered board format. Enzyme conjugated avidin, straptavidin:HRP, was used at a concentration of 1:5,000 in diluting solution. Finally, 100 μl substrate solution (0.05 M citrate buffer containing 0.08 % OPD and 30 % H2O2) was added to each well, and kept at room temperature. Optical density was taken at 492 nm in ELISA reader (Tecan Sunrise, Austria) after 5 min. The results were expressed as stimulation indices (SI) using the following formula: mean of the optical density obtained from the stimulated culture/the mean of the optical density obtained from negative control culture.
Monitoring of immunological response
The humoral immune response to rHaa86 antigen was monitored by indirect ELISA. The ELISA was optimized and antigen was applied to the microtiter plate in a concentration of 6 μg/ml. The collected sera were diluted into 1:500 and were used in triplicate wells. Sheep anti-bovine IgG, IgG1 and IgG2:HRPO conjugate (AbD serotec, UK) were used at a dilution of 1:10,000 as secondary antibody. The peroxidase mediated colour development was performed at room temperature with o-phenylene diamine dihydrochloride (OPD) (Pierce, USA) in citrate buffer, pH 5.0. The reaction was stopped with 50 μl of 3 N HCl per well, and absorbance was recorded by microplate ELISA reader (Tecan-Sunrise, Austria), as the mean OD492 of triplicate samples.
Statistical analysis
The analysis of variance was used for comparing the data of cytokine and humoral immune responses among the different groups of the experimental calves and between different days within the same group of calves. Significance at 5 % level (P < 0.05) was used to define differences in different parameters.
Results
Gel purification of rHaa86
The purity and integrity of gel eluted protein was confirmed (Fig. 1) and the recovery of the pure protein was about 40–50 %.
Whole blood culture
After 30 h of incubation the consistency and colour of blood was found normal in culture plate. The sterility, integrity of blood cells and changes in lymphocytes (blast formation), were conformed on microscopic examination. Trypan blue staining of lymphocytes showed that more than 80 % lymphocytes were viable.
Interferon-γ response in in vitro lymphocyte stimulation
The following ELISA condition was standardized as optimum for IFN-γ assay on the basis of differences of optical densities recorded amongst various concentration of rIFN-γ used as positive and without rIFN-γ as negative control: Concentration of mouse anti-bovine IFN-γ:biotin 03 μg/ml, IFN-γ detection up to 20 pg/ml, dilution of culture supernatant 1:10 and the mouse anti-bovine IFN-γ and straptavidin: HRP conjugate were used in 5 μg/ml and 5000−1 dilution, respectively, as per the manufacturer’s instructions. The standard graph of bovine recombinant IFN-γ was plotted (Fig. 2) and a significant difference in SI and OD value up to 200 pg/ml was recorded. The lowest detectable value of IFN-γ was 20 pg/ml and SI at this level was 1.16 which was greater than maximum SI calculated from individual calf. The pattern of expression of IFN-γ was shown in Fig. 3. The SI of all the groups of animals were within a range of 1–1.14 and there were no significant differences in IFN-γ expression amongst the animals of all the groups.
Antibody response in calf
Groups 1
The whole IgG, IgG1 and IgG2 responses of the group were pooled and depicted in Fig. 4. The mean OD492 values of the serum samples rose to 3.8, 3.7 and 2.3 times as against the pre-immunized value on day 21 of primary immunization, and 7.0, 8.0 and 4.0 times of the pre-immunized value on day 75 post primary immunization for IgG, IgG1 and IgG2, respectively. The sustained antibody levels were detected till 60 days of last immunization (day 120). On day 140 post immunization, a decreasing trends was recorded. In comparison to IgG and IgG1, the animal to animal variation at IgG2 response was at very high level (2.5 to 5.4 times) and overall response was not significant.
Groups 2 and 3
The mean OD values of the serum samples of the calves were recorded in the range of 0.081 ± 001 to 0.090 ± 0.003 for IgG, 0.071 ± 0.001 to 0.077 ± 0.002 for IgG1 and 0.050 ± 0.002 to 0.063 ± 0.001 for IgG2 during the entire period of experiment.
Discussion
Immunization with concealed antigens of ticks was attempted with an idea to induce antibody production to molecules of ticks performing essential physiological functions. It was assumed that antibodies would enter the body of ticks through blood and disrupt the essential functions of the tick with subsequent killing. It is well established that T helper cells are required for optimal isotype switching and the production of multiple antibody subclasses during antigen specific immune responses (Mond et al. 1995; Snapper et al. 1993; Laman et al. 1996). Clery et al. (1996) recorded no to low levels of expression of IFN-γ protein and predominant IgG1 response in cattle experimentally infected with the helminth parasite, Fasciola hepatica. He correlated the lack or relatively low levels of serum IgG2 antibody in infected cattle with a relatively weak IFN-γ response. In many of the immunization study using tick antigens the role of humoral antibody response in conferring immunity against challenge infestation has been established (Allen 1989). In earlier experiment, the immune response against rHaa86 (Azhahianambi et al. 2009a, b; Jeyabal et al. 2010), Bm86 (Odongo et al. 2007) and Bm91 (Lambertz et al. 2012) were monitored by whole IgG measurement in rHaa86, Bm86 and Bm91 immunized cattle, respectively. The immunized animals developed a strong and specific humoral immune response characterized by high anti-Haa86, Bm86 and Bm91 IgG level. Accordingly, to trace the mechanism involved in conferring immunity in ticks following immunization with rHaa86, both IgG1 and IgG2 response was characterized. The IgG1 response was dominated over IgG2 response throughout the period of experiment. As reported by other workers the IFN-γ response never reached at significant level throughout the period of experiments. Since IgG2 and IFN-γ are interlinked, the Th2 response is established as possible mode of mechanism of conferring antibody mediated protection against challenged ticks. In the present experiment, the antibody response was characterized specifically against rHaa86 and the protocol can be used for antibody monitoring using different recombinant proteins of parasite origin.
References
Allen JR (1989) Immunology, immunopathology, and immunoprophylaxis of tick and mite infestations. In: Soulsby EJL (ed) Immune responses in parasitic infections: immunology, immunopathology and immunoprophylaxis, vol 4. CRC Press, Boca Raton
Azhahianambi P, Ray DD, Chaudhuri P, Gupta R, Ghosh S (2009a) Vaccine efficacy of Bm86 ortholog of hyalomma anatolicum anatolicum rHaa86 expressed in prokaryotic expression system. J Parasitol res. doi:10.1155/2009/165812
Azhahianambi P, Suryanarayana VVS, Ghosh S (2009b) Cloning expression and immunoprotective efficacy of rHaa86, the homologue of the Bm86 tick vaccine antigen, from Hyalomma anatolicum anatolicum. Parasite Immunol 31:111–122
Clery D, Torgerson P, Mulcahy G (1996) Immune responses of chronically infected adult cattle to Fasciola hepatica. Vet Parasitol 62:71–76
Cobon GS, Willadsen P (1990) Vaccine to prevent cattle tick infestations in new generation vaccine. Woodrow, Levine and Marcel, New York 901
Estes DM, Brown WC (2002) Type 1 and type 2 responses in regulation of Ig isotype expression in cattle. Vet Immunol Immunopathol 90:1–10
Estes DM, Closser NM, Allen GK (1994) IFN-gamma stimulates IgG2 production from bovine B cells co-stimulated with anti-mu and mitogen. Cell Immunol 154:287–295
Estes DM, Hirano A, Heussler VT, Dobbelaere DA, Brown WC (1995) Expression and biological activities of bovine interleukin-4: effects of recombinant bovine interleukin-4 on T cell proliferation and B cell differentiation and proliferation in vitro. Cell Immunol 163:268–273
Ghosh S, Singh NK, Das G (2005) Assessment of duration of immunity in crossbred cattle immunized with glycoproteins isolated from Hyalomma anatolicum anatolicum and Boophilus microplus. Parasitol Res 95:319–326
Hasvold HJ, Valheim M, Berntsen G, Storset AK (2002) In vitro responses to purified protein derivate of caprine T lymphocytes following vaccination with live strains of Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunopathol 90:79–89
Jeyabal L, Azhahianambi P, Susitha K, Ray DD, Chaudhuri P, Vanlahmuaka, Ghosh S (2010) Efficacy of rHaa86, an orthologue of Bm86, against challenge infestations of Hyalomma anatolicum anatolicum. Transbound Emerg Dis 57:96–102
Kemp DH, Agbede RIS, Johnston LAY, Gough JM (1986) Immunisation of cattle against Boophilus microplus using extract derived from adult female ticks: feeding and survival of the parasite on the vaccinated cattle. Int J Parasitol 16:115–119
Kumar B, Azhahianambi P, Ray DD, Chaudhuri P, de la Fuente J, Kumar R, Ghosh S (2012) Comparative efficacy of rHaa86 and rBm86 against Hyalomma anatolicum anatolicum and Rhipicephalus (Boophilus) microplus. Immuno Parasitol 34:297–301
Laman JD, Claassen E, Noelle RJ (1996) Functions of CD40 and its ligand, GP39 (CD40L). Crit Rev Immunol 16:59–65
Lambertz C, Chongkasikit N, Jittapalapong S, Gauly M (2012) Immune response of Bos indicus cattle against the anti-tick antigen Bm91 derived from local Rhipicephalus (Boophilus) microplus ticks and its effect on tick reproduction under natural infestation. J Parasitol Res. Article (ID) 907607. doi:10.1155/2012/907607
Mond JJ, Lees A, Snapper CM (1995) T cell independent antigens type 2. Annu Rev Immunol 13:655–661
Odongo D, Kamau L, Skilton R, Mwaura S, Nitsch C, Musoke A, Taracha E, Daubenberger C, Bishop R (2007) Vaccination of cattle with TickGARD induce corss-reactive antibodies binding to conserved linear peptides of Bm86 homologues in Boophilus decoloratus. Vaccine 25:1287–1296
Rand KN, Moore T, Sriskantha A, Spring K, Tellam R, Willadsen P, Cobon SG (1989) Cloning and expression of a protective antigen from the cattle tick Boophilus microplus. Proc National Acad Sci 86:9657–9661
Rodriguez M, Rubiera R, Penichet M, Montesions R, Cremata J, Falcon V, Sanchez G, Bringas R, Cordoves C, Valdes M, Lleonart R, Herrera L, de la Fuente J (1994) High level expression of the Boophilus microplus Bm86 antigen in the yeast Pichia pastoris forming highly immunogenic particles for cattle. J Biotecnol 33:135–146
Singh NK, Ghosh S (2003) Experimental immunization of crossbred cattle with glycoproteins isolated from the larvae of Hyalomma anatolicum anatolicum. Exp Appl Acarol 31:297–314
Snapper CM, Waegell W, Beernink H, Dasch JR (1993) Transforming growth factor-beta 1 is required for secretion of IgG of all subclasses by LPS-activated murine B cells in vitro. J Immunol 151:4625–4636
Talwar GP, Gupta SK (1992) A hand book of practical and clinical immunology, vol 1. CBS Publishers and distributors, Delhi, pp 219–232
Weynants V, Godfroid J, Limbourg B, Saegerman C, Letesson JJ (1995) Specific bovine brucellosis diagnosis based on in vitro antigen-specific gamma interferon production. J Clin Microbiol 33:706–712
Willadsen P, McKenna RV, Riding GA (1988) Isolation from the cattle tick Boophilus microplus, of antigenic material capable of eliciting a protective immunological response in the bovine host. Int J Parasitol 18:183–188
Willadsen P, Riding G, Mckenna RV, Kemp DH, Tellam RL, Nielsen JN, Lahnstein J, Cobon GS, Gough JM (1989) Immunological control of the parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J Immunol 143:1346–1351
Acknowledgments
This work has been facilitated through the Integrated Consortium on Ticks and Tick-borne Diseases (ICTTD-3), financed by the International Cooperation Programme of the European Union through Coordination Action Project no. 510561.The contribution made by the laboratory staff (Mr. Laxmi Lal, Naresh Kumar and Mohan Lal) is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, B., Ray, D.D. & Ghosh, S. Immune responses against rHaa86 in cross-bred cattle. J Parasit Dis 39, 292–297 (2015). https://doi.org/10.1007/s12639-013-0347-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-013-0347-9