Abstract
Introduction
The Glidescope® video-laryngoscopy appears to provide better glottic visualization than direct laryngoscopy. However, it remains unclear if it translates into increased success with intubation.
Methods
We systematically searched electronic databases, conference abstracts, and article references. We included trials in humans comparing Glidescope® video-laryngoscopy to direct laryngoscopy regarding the glottic view, successful first-attempt intubation, and time to intubation. We generated pooled risk ratios or weighted mean differences across studies. Meta-regression was used to explore heterogeneity based on operator expertise and intubation difficulty.
Results
We included 17 trials with a total of 1,998 patients. The pooled relative risk (RR) of grade 1 laryngoscopy (vs ≥ grade 2) for the Glidescope® was 2.0 [95% confidence interval (CI) 1.5 to 2.5]. Significant heterogeneity was partially explained by intubation difficulty using meta-regression analysis (P = 0.003). The pooled RR for nondifficult intubations of grade 1 laryngoscopy (vs ≥ grade 2) was 1.5 (95% CI 1.2 to 1.9), and for difficult intubations it was 3.5 (95% CI 2.3 to 5.5). There was no difference between the Glidescope® and the direct laryngoscope regarding successful first-attempt intubation or time to intubation, although there was significant heterogeneity in both of these outcomes. In the two studies examining nonexperts, successful first-attempt intubation (RR 1.8, 95% CI 1.4 to 2.4) and time to intubation (weighted mean difference −43 sec, 95% CI −72 to −14 sec) were improved using the Glidescope®. These benefits were not seen with experts.
Conclusion
Compared to direct laryngoscopy, Glidescope® video-laryngoscopy is associated with improved glottic visualization, particularly in patients with potential or simulated difficult airways.
Résumé
Introduction
Le vidéolaryngoscope Glidescope® semble procurer une meilleure visualisation de la glotte que la laryngoscopie directe. Il n’est toutefois pas certain que cela se traduise par une meilleure réussite des intubations.
Méthodes
Nous avons fait une recherche systématique dans les bases de données électroniques, parmi les résumés de congrès et les références d’articles. Nous avons inclus les études chez l’homme comparant le vidéolaryngoscope Glidescope® à la laryngoscopie directe pour ce qui concerne la visualisation de la glotte, la réussite de l’intubation au premier essai et le délai d’intubation. Nous avons généré un risque relatif global ou des différences moyennes pondérées entre les études. Une métarégression a permis d’explorer l’hétérogénéité en fonction de l’expertise de l’opérateur et de la difficulté d’intubation.
Résultats
Nous avons inclus 17 études incluant un total de 1998 patients. Le risque relatif (RR) global d’une laryngoscopie de grade 1 (contre une laryngoscopie de grade ≥ 2) avec le Glidescope® a été de 2,0 (intervalle de confiance [IC] à 95 % : 1,5 à 2,5). L’hétérogénéité significative a été expliquée en partie par la difficulté d’intubation en utilisant l’analyse par métarégression (P = 0,003). Le RR global pour les intubations non difficiles de grade 1 à la laryngoscopie (contre les grades ≥ 2) a été de 1,5 (IC à 95 % : 1,2 à 1,9) et le RR pour les intubations difficiles a été de 3,5 (IC à 95 % : 2,3 à 5,5). Il n’y a pas eu de différence entre le Glidescope® et la laryngoscopie directe pour ce qui concerne l’intubation réussie au premier essai ou pour le délai d’intubation, bien qu’une hétérogénéité significative ait été observée pour ces deux critères d’évaluation. Dans les deux études impliquant des non-experts, la première tentative réussie d’intubation (RR: 1,8; IC à 95 % : 1,4 à 2,4) et le délai d’intubation (différence de moyenne pondérée −43 sec; IC à 95 % : −72 à −14 sec) ont été améliorés par l’utilisation du Glidescope®. Ces avantages n’ont pas été retrouvés chez les experts.
Conclusion
Comparée à la laryngoscopie directe, la vidéolaryngoscopie avec le Glidescope® est associée à une amélioration de la visualisation de la glotte, en particulier chez les patients avec des voies aériennes difficiles potentielles ou simulées.
Similar content being viewed by others
Anesthesiologists perform endotracheal intubation (ETI) in the operating room under controlled circumstances, and the procedure carries a low risk of complications.1 Although laryngoscopy is difficult in 6-10% of intubations,2 - 4 difficult or failed intubations are much less frequent, occurring in 1.8-5.8% and 0.13-0.30%, respectively.2 , 5 - 8 Unfortunately, physical findings on examination of the airway discriminate poorly between potentially easy and difficult intubations.9 Thus, anesthesiologists need to be prepared for the unanticipated difficult airway, as many of these patients have had a “reassuring” airway physical examination. In addition to the unanticipated difficult airway, there are circumstances that lend themselves to a high risk of difficult laryngoscopy and tracheal intubation. In particular, emergent ETI outside of the operating theatre is associated with a much higher risk of difficult laryngoscopy and intubation.10 - 13 As such, techniques that may improve successful intubation may be especially beneficial in these emergent environments. Laryngoscopy with the Glidescope® video-laryngoscope (Verathon Medical, Bothell, WA, USA) appears to be associated with improved glottic visualization.14 , 15 Whether the improved visualization translates into increased success at ETI, when compared to direct laryngoscopy, remains unclear.14 , 16 Given this uncertainty, our goal was to perform a systematic review and meta-analysis of randomized and quasi-randomized trials comparing Glidescope® video-laryngoscopy to direct laryngoscopy regarding glottic visualization, successful first-attempt intubation, and time to intubation. In addition, we explored the heterogeneity in these outcomes based on operator expertise and according to the difficulty of the intubation.
Methods
This article reports our meta-analysis of controlled trials of Glidescope® video-laryngoscopy compared to direct laryngoscopy in accordance with the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) statement.17 A review protocol was not published for this study.
Search strategy
We systematically searched MEDLINE (1966 to June 13, 2011), EMBASE (1977 to June 13, 2011), and The Cochrane Central Register of Controlled Trials (CENTRAL) (1948 to June13, 2011) for randomized and quasi-randomized trials comparing Glidescope® video-laryngoscopy to direct laryngoscopy regarding the glottic view, successful first-attempt intubation, and time to intubation. We included non-English publications. We hand-searched abstracts of selected conferences from 2000 to 2010, including those of the American Society of Anesthesiologists, the Canadian Anesthesiologists’ Society, and the International Anesthesia Research Society. We also hand-searched bibliographies of all relevant trials and review articles.
For the bibliographic review, we constructed search filters for the concepts “Glidescope video-laryngoscope” and “clinical trials” using a combination of exploded Medical Subject Heading (MeSH) terms and text words all combined with the Boolean operator “OR.” The Glidescope® video-laryngoscope filter contained the text words glidescope and video-laryngoscope. The clinical trials filter included the MeSH terms clinical trials [publication type], clinical trials as topic, placebos with text words trial*, random* or placebo. A similar search strategy was used for both EMBASE and CENTRAL.
Selection criteria, data abstraction, and methodological quality
In duplicate and independently, two authors (D.G., D.L.) screened all articles and abstracts, which were included if they 1) were randomized or quasi-randomized controlled trials, 2) compared direct laryngoscopy to Glidescope® video-laryngoscopy, 3) addressed adult patients, and 4) contained any outcome of interest (Cormack-Lehane view,18 successful first-attempt intubation, time to intubation).
The same two authors abstracted the data and assessed the study quality in duplicate and independently. Disagreement was resolved by discussion and arbitrated if necessary by a third author (P.C.). We abstracted the year of publication, sample size, country of origin, operator training and experience, physical examination of the airway, anticipated or history of difficult intubation, application of manual in-line stabilization, Cormack-Lehane grade, successful first attempt at intubation, and time required to intubate. We contacted investigators for missing data as necessary.
Statistical analysis
We used relative risk (RR) as the summary measure for dichotomous outcomes (glottic view and successful first intubation attempt) and the weighted mean difference (WMD), in seconds, as the summary measure for time to intubate. We applied a half-integer continuity correction to all four cells if the event rates were zero. The random effects method of DerSimonian and Laird was used to generate a pooled RR or WMD across studies.19 Random effects analysis yields a more conservative estimate than the fixed-effects model in the presence of between-study heterogeneity. We assessed statistical heterogeneity using Cochran’s Q statistic20 (with P < 0.10 considered significant) and expressed the quantity using the I 2 statistic and 95% confidence interval (CI). The I 2 statistic indicates the percentage of variation in study results that is due to between-study heterogeneity rather than sampling variability.21 We assessed for the following outcomes: Cormack-Lehane view grade 1 vs grade ≥ 2, successful first-attempt intubation, and time to intubate (in seconds).
Sources of potential heterogeneity identified a priori were the experience level of the operator (anesthesia or casualty consultants or house staff vs “other”) and potential difficulty. Intubations were considered difficult in studies that included patients with a known prior difficult intubation, physical examination features suggesting a difficult intubation, or in whom difficult intubation was simulated by providing manual-in-line stabilization. Random-effects meta-regression was used to evaluate the relation between these subgroups on the final pooled estimates.22 We evaluated the presence of publication bias by visual inspection of the funnel plot and by using Egger’s and Begg’s tests, with P < 0.05 considered statistically significant. All analyses were done using Stata 10.0 (2007) (StataCorp LP, College Station, TX, USA).
Results
Literature search
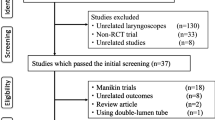
A total of 297 citations were identified during the bibliographic search: 76 from MEDLINE, 150 from EMBASE, and 71 from CENTRAL. We excluded 264 citations on the initial abstract screen (178 duplicate citations, 86 from screening). We identified three published abstracts from conference screening and five citations from reference lists. This resulted in 41 citations for full text review. The exclusion of 24 citations (for reasons listed in Fig. 1) resulted in 17 trials being included in the current analysis.14 - 16 , 23 - 36 We contacted one author, who provided the raw data for the number of attempts required for intubation, which was not included in the published article.25
Study characteristics
Table 1 lists the trial characteristics. Of the 17 included trials with a total of 1,998 subjects, three were published abstracts.33 - 35 One trial was published in Japanese.36 Although most of the studies randomized subjects to Glidescope® video-laryngoscopy vs direct laryngoscopy, in four studies subjects underwent both techniques sequentially, with the order of the techniques allocated randomly.24 , 29 , 31 , 33 The operators in most of the studies were anesthesiologists experienced with both techniques. There were two studies in which the primary operators were inexperienced personnel consisting of nonanesthesia house staff36 or trainees consisting of paramedics, nurses, and medical students.14 Although the trial by Jones and colleagues included anesthesia consultants and residents, only 39% were experienced with the Glidescope® (≥ 10 intubations).16 In contrast to all the other studies of elective patients in the operating theatre, the trial by Yeatts et al. examined patients presenting to the casualty department.35
Most of the studies specifically excluded patients with a known or anticipated difficult airway.14 , 16 , 23 , 25 , 27 , 28 , 30 - 34 In contrast, two studies selected patients with clinical examination features suggesting a difficult intubation.24 , 26 Five studies attempted to increase the difficulty of laryngoscopy by applying manual in-line stabilization.23 , 28 , 29 , 31 , 34 Finally, three studies did not specify any exclusion or inclusion criteria based on prior or anticipated difficulty of laryngoscopy.15 , 35 , 36
Grade 1 glottic view
Twelve studies presented outcomes corresponding to our primary outcome, glottis visualization (Table 2).14 - 16 , 23 , 24 , 26 - 29 , 32 - 34 A forest plot is presented in Fig. 2. The pooled RR across all studies was 2.0 (95% CI 1.5 to 2.5, P < 0.001), indicating improved glottic visualization using the Glidescope® when compared to the direct laryngoscope. There was significant between-study heterogeneity in our primary analysis (Q = 74.8, df = 11, P < 0.001), with a corresponding I 2 statistic of 85% (95% CI 76 to 91). Only one study used inexperienced operators14; thus, we were unable to explore heterogeneity by expertise. We examined for effect modification by anticipated or simulated difficult laryngoscopy (manual in-line stabilization). Meta-regression demonstrated that the benefit to glottic visualization afforded by Glidescope® was even more pronounced in studies that considered patients with anticipated or simulated difficult airways (P = 0.003). The resultant pooled estimates were as follows: for nondifficult intubations (RR 1.5, 95% CI 1.2 to 1.9) and for difficult intubations (RR 3.5, 95% CI 2.3 to 5.5). Visual inspection of the funnel plot revealed an absence of small studies favouring direct laryngoscopy (not shown). This publication bias was confirmed on Begg’s (P = 0.04) and Egger’s (P = 0.07) regression testing.
Risk ratios (RR) of Cormack-Lehane (CL) grade 1 (vs ≥ grade 2) in clinical trials comparing Glidescope® video-laryngoscopy to direct laryngoscopy stratified by the difficulty of the intubation. Subjects were considered to have difficult intubations in studies that included patients with known prior difficult intubation, physical examination features suggesting difficult intubation, or in which difficult intubation was simulated by providing manual-in-line stabilization. The pooled estimate was derived using the DerSimonian and Laird random effects method with grey squares depicting individual study point estimates of the RR. Larger squares indicate a larger weight of the study when calculating the pooled estimate. Solid horizontal lines display the 95% confidence interval (CI) of the point estimate. Dashed vertical line represents an RR of 1.00, indicating no difference between Glidescope® video-laryngoscopy and direct laryngoscopy. Solid vertical lines represent the pooled estimates. Test for heterogeneity was significant using meta-regression analysis (P = 0.003). DL = direct laryngoscopy; GS = Glidescope®
Successful first-attempt intubation
Fourteen studies presented data on intubation success (Table 2).14 - 16 , 23 - 28 , 30 , 32 , 33 , 35 , 36 A forest plot is presented in Fig. 3. The pooled RR across studies was 1.1 (95% CI 0.99 to 1.2, P = 0.09). There was significant between-study heterogeneity (Q = 117.12, df = 13, P < 0.001), with a corresponding I 2 statistic of 89% (95% CI 83 to 93). Two studies presented data on inexperienced operators,14 , 36 and meta-regression demonstrated effect modification by operator expertise (P = 0.001). Compared to the direct laryngoscope, the Glidescope® increased the success of first intubation attempts in studies with nonexpert operators (RR 1.8, 95% CI 1.4 to 2.4) but not amongst airway experts (RR 1.0, 95% CI 0.94 to 1.20). There was no effect measure modification by potential or simulated difficult airways (P = 0.89). There was no evidence of publication bias on this outcome by Begg’s (P = 0.38) or Egger’s (P = 0.86) testing.
Risk ratios (RR) of successful first-attempt intubation in clinical trials comparing Glidescope® video-laryngoscopy to direct laryngoscopy stratified by operator expertise (anesthesia or casualty consultants or house staff vs “other”). The pooled estimate was derived using the DerSimonian and Laird random effects method with grey squares depicting individual study point estimates of the RR. Larger squares indicate a larger weight of the study when calculating the pooled estimate. Solid horizontal lines display the 95% CI of the point estimate. Dashed vertical line represents an RR of 1.00, indicating no difference between Glidescope® video-laryngoscopy and direct laryngoscopy. Solid vertical lines represent the pooled estimates. Test for heterogeneity by operator expertise was significant using meta-regression analysis (P = 0.001). DL = direct laryngoscopy; GS = Glidescope®
Time to intubation
The time required to intubate was available in 15 studies (Table 2).14 - 16 , 23 - 28 , 30 - 32 , 34 - 36 A forest plot is presented in Fig. 4. The pooled WMD across studies did not differ between Glidescope® video-laryngoscopy and direct laryngoscopy (WMD 3.8 sec, 95% CI −1.7 to 9.3 sec, P = 0.17). However, there was significant between-study heterogeneity in these results (Q = 675.7, df = 14, P < 0.001) with an I 2 statistic of 98% (95% CI 97 to 98) that was not explained by the difficulty of the intubation on meta-regression (P = 0.85). Meta-regression did demonstrate that operator expertise explained some of the between-study heterogeneity observed (P = 0.004), with the Glidescope® being associated with a shorter time to intubation in the two studies with nonexperts as the primary operators (WMD −43 sec, 95% CI −72 to −14 sec). There was no difference in time to intubation amongst experts (WMD 8 sec, 95% CI −2 to 17 sec). There was no effect measure modification by airway difficulty on meta-regression (P = 0.74). There was no evidence of publication bias on this outcome by Begg’s (P = 0.18) or Egger’s (P = 0.96) testing.
Weighted mean difference (WMD), in seconds, in clinical trials comparing Glidescope® video-laryngoscopy to direct laryngoscopy stratified by operator expertise (anesthesia or casualty consultants or housestaff vs “other”). The pooled estimate was derived using the DerSimonian and Laird random effects method with grey squares depicting an individual study point estimate of the mean difference. Larger squares indicate a larger weight of the study when calculating the pooled estimate. Solid horizontal lines display the 95% CI of the point estimate. Dashed vertical line represents a WMD of 0, indicating no difference between Glidescope® video-laryngoscopy and direct laryngoscopy. Solid vertical lines represent the pooled estimate. Test for heterogeneity by operator expertise was significant using meta-regression analysis (P = 0.004)
Discussion
In this meta-analysis of randomized trials comparing Glidescope® video-laryngoscopy to direct laryngoscopy, the former was associated with improved glottic visualization, particularly amongst studies that considered patients with potential or simulated difficult airways. Although there was an improved successful first intubation attempt and faster time to intubation with Glidescope® video-laryngoscopy, it was confined to studies of nonexpert operators. There was no benefit in either of these outcomes in studies with expert operators. Importantly, there was marked between-study heterogeneity in all three outcomes.
Improved glottic visualization (compared to that with direct laryngoscopy) is a consistent finding with nonstandard laryngoscopes, including video-laryngoscopes.37 Building on this, we have demonstrated that improvement in glottic visualization afforded by the Glidescope® is even greater in studies using patients with either simulated (via manual in-line stabilization) or physical examination predictors of difficult laryngoscopy. This is not surprising as the Glidescope® appears to be used often by clinicians in these situations. A large observation cohort study by Aziz and colleagues of 2,004 Glidescope® intubations showed that most were performed in patients with clinical examination predictors of a difficult direct laryngoscopy.38 Thus, clinicians are triaging patients to video-laryngoscopy when difficulty with endotracheal intubation is anticipated.
As in our current review, a prior systematic review demonstrated significant heterogeneity when comparing the Glidescope® results to those achieved with the direct laryngoscope.37 In contrast, we attempted to quantify and evaluate sources of heterogeneity by both operator expertise and potential difficulty of the intubation. Given that most of the studies were performed by airway management experts on patients without predictors of difficult intubation, it is not surprising that the Glidescope® did not result in improved first-attempt success. Aside from one trial with a markedly low rate of 63%, documented by Morello et al.,33 the rest of the studies with experts—and excluding difficult airways—had a first-attempt success rate of > 90%.15 , 16 , 27 , 32 This high rate of success with direct laryngoscopy by anesthesiologists is reflected in other clinical studies.6 Even in the unlikely scenario that Glidescope® video-laryngoscopy would improve the success rate in patients without difficult airways by experts, it would require a large sample of patients to prove it. Thus, potential benefits of Glidescope® video-laryngoscopy may lie with: 1) use in patients with clinical features indicating difficult laryngoscopy; 2) it being used as a rescue method following failed direct laryngoscopy; or 3) it being used by nonexpert providers. Indeed, the observational study by Aziz et al. demonstrated that the Glidescope® was successful in 96% of patients with predictors of difficult direct laryngoscopy and in 94% following failed direct laryngoscopy.38
Although our review did show increased first-attempt success and decreased time to intubation in studies of nonexperts with the Glidescope® compared to direct laryngoscopy, these results must be interpreted with caution given that there were only two studies in this subgroup.14 , 36 Rather, the possible benefit of Glidescope® video-laryngoscopy amongst nonexperts should be viewed as an area that requires further research.
This systematic review and meta-analysis highlights several areas that need to be addressed. How is expertise developed and defined, particularly when a new technology is introduced? What role should nonexperts play in airway management? Studies examining new technology are prone to proficiency bias. Despite this fact, anesthesiologists have incorporated the Glidescope® into their armamentarium with a high rate of success.39 Although it seems reasonable to assume that anesthesia consultants are experts, it remains less clear how, and at what point, this competence develops. When examining trainees, we have previously shown that anesthesia house staff were successful in 85% of their first attempts at intubating critically ill patients.40 This success rate is very respectable given that this is a population with a 6.6-22.0% risk of a difficult intubation.11 , 13 , 41 Furthermore, anesthesia house staff require fewer attempts to perform tracheal intubation compared to their nonanesthesia counterparts. Having an airway management expert at the bedside for each intubation may be advantageous, but there are many situations when this is not feasible. In many environments, there may be limited, if any, access to anesthesiologists, and airway management must be delivered by physicians from different speciality backgrounds. Endotracheal intubation remains a competence objective of the Royal College of Physicians and Surgeons of Canada in training for internal medicine.42 Also, use of an advanced airway (e.g., endotracheal tube) remains a fundamental skill in Advanced Cardiac Life Support according to the 2005 American Heart Association Guidelines.43 Thus, technologies that can improve the success of airway management, particularly in the hands of nonexperts, are desirable and should be studied. An example is Glidescope® use by prehospital paramedics.44
There are several limitations to our review. As previously stated, there was marked heterogeneity in all of our endpoints that was only partially explained by subgroup analysis. We attempted to account for this heterogeneity by performing a random-effects meta-regression, which yields a more conservative pooled estimate when between-study heterogeneity exists.45 In addition, we explored heterogeneity by a priori defined subgroups and presented these results when they were significant. As with all meta-analyses, our review is subject to information bias. We defined expertise and difficulty a priori, but there may be marked differences between studies with respect to subject or operator characteristics that we were unable to evaluate from the available information. Another limitation is the low number of studies that included nonexperts, which markedly limits the ability to evaluate the effect of video-laryngoscopy in this important subgroup. Finally, there was evidence of publication bias in our primary outcome of the glottic view, suggesting that small studies favouring direct laryngoscopy were not being published. However, tests of publication bias are subject to a high risk of a type I error in the presence of significant heterogeneity, limiting their interpretability.46
In conclusion, we have shown in our meta-analysis that, compared to direct laryngoscopy, Glidescope® video-laryngoscopy is associated with improved glottic visualization, particularly in studies that considered patients with potential or simulated difficult airways. In addition, there is marked heterogeneity in all of our outcomes that is partially explained by operator expertise or the difficulty of intubation. There is a need for further evaluation of potential improvements in successful first-attempt intubations or time to intubate among nonexperts.
References
Cheney FW, Posner KL, Lee LA, Caplan RA, Domino KB. Trends in anesthesia-related death and brain damage: a closed claims analysis. Anesthesiology 2006; 105: 1081-6.
Rose DK, Cohen MM. The incidence of airway problems depends on the definition used. Can J Anaesth 1996; 43: 30-4.
Ezri T, Weisenberg M, Khazin V, et al. Difficult laryngoscopy: incidence and predictors in patients undergoing coronary artery bypass surgery versus general surgery patients. J Cardiothorac Vasc Anesth 2003; 17: 321-4.
el-Ganzouri AR, McCarthy RJ, Tuman KJ, Tanck EN, Ivankovich AD. Preoperative airway assessment: predictive value of a multivariate risk index. Anesth Analg 1996; 82: 1197-204.
Crosby ET, Cooper RM, Douglas MJ, et al. The unanticipated difficult airway with recommendations for management. Can J Anaesth 1998; 45: 757-76.
Karkouti K, Rose DK, Wigglesworth D, Cohen MM. Predicting difficult intubation: a multivariable analysis. Can J Anesth 2000; 47: 730-9.
Rose DK, Cohen MM. The airway: problems and predictions in 18, 500 patients. Can J Anaesth 1994; 41: 372-83.
Rocke DA, Murray WB, Rout CC, Gouws E. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology 1992; 77: 67-73.
Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. Anesthesiology 2005; 103: 429-37.
Graham CA, Beard D, Henry JM, McKeown DW. Rapid sequence intubation of trauma patients in Scotland. J Trauma 2004; 56: 1123-6.
Griesdale DE, Bosma TL, Kurth T, Isac G, Chittock DR. Complications of endotracheal intubation in the critically ill. Intensive Care Med 2008; 34: 1835-42.
Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults. A prospective investigation of 297 tracheal intubations. Anesthesiology 1995; 82: 367-76.
Benedetto WJ, Hess DR, Gettings E, et al. Urgent tracheal intubation in general hospital units: an observational study. J Clin Anesth 2007; 19: 20-4.
Nouruzi-Sedeh P, Schumann M, Groeben H. Laryngoscopy via Macintosh blade versus GlideScope: success rate and time for endotracheal intubation in untrained medical personnel. Anesthesiology 2009; 110: 32-7.
Sun DA, Warriner CB, Parsons DG, Klein R, Umedaly HS, Moult M. The GlideScope video laryngoscope: randomized clinical trial in 200 patients. Br J Anaesth 2005; 94: 381-4.
Jones PM, Armstrong KP, Armstrong PM, et al. A comparison of GlideScope videolaryngoscopy to direct laryngoscopy for nasotracheal intubation. Anesth Analg 2008; 107: 144-8.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews, meta-analyses of studies that evaluate healthcare interventions: explanation, elaboration. BMJ 2009; 339: b2700.
Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia 1984; 39: 1105-11.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177-88.
Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10: 101-29.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557-60.
Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002; 21: 1559-73.
Lim Y, Yeo SW. A comparison of the GlideScope with the Macintosh laryngoscope for tracheal intubation in patients with simulated difficult airway. Anaesth Intensive Care 2005; 33: 243-7.
Serocki G, Bein B, Scholz J, Dorges V. Management of the predicted difficult airway: a comparison of conventional blade laryngoscopy with video-assisted blade laryngoscopy and the GlideScope. Eur J Anaesthesiol 2010; 27: 24-30.
Siddiqui N, Katznelson R, Friedman Z. Heart rate/blood pressure response and airway morbidity following tracheal intubation with direct laryngoscopy, GlideScope and trachlight: a randomized control trial. Eur J Anaesthesiol 2009; 26: 740-5.
Malik MA, Subramaniam R, Maharaj CH, Harte BH, Laffey JG. Randomized controlled trial of the Pentax AWS, Glidescope, and Macintosh laryngoscopes in predicted difficult intubation. Br J Anaesth 2009; 103: 761-8.
Teoh WH, Shah MK, Sia AT. Randomised comparison of Pentax AirwayScope and Glidescope for tracheal intubation in patients with normal airway anatomy. Anaesthesia 2009; 64: 1125-9.
Malik MA, Maharaj CH, Harte BH, Laffey JG. Comparison of Macintosh, Truview EVO2, Glidescope, and Airwayscope laryngoscope use in patients with cervical spine immobilization. Br J Anaesth 2008; 101: 723-30.
Robitaille A, Williams SR, Tremblay MH, Guilbert F, Theriault M, Drolet P. Cervical spine motion during tracheal intubation with manual in-line stabilization: direct laryngoscopy versus GlideScope videolaryngoscopy. Anesth Analg 2008; 106: 935-41.
Xue FS, Zhang GH, Li XY, et al. Comparison of hemodynamic responses to orotracheal intubation with the GlideScope videolaryngoscope and the Macintosh direct laryngoscope. J Clin Anesth 2007; 19: 245-50.
Turkstra TP, Craen RA, Pelz DM, Gelb AW. Cervical spine motion: a fluoroscopic comparison during intubation with lighted stylet, GlideScope, and Macintosh laryngoscope. Anesth Analg 2005; 101: 910-5.
Bilehjani E, Fakhari S. Hemodynamic response to laryngoscopy in ischemic heart disease: Macintosh blade versus Glidescope videolaryngoscope. Rawal Med J 2009; 34: 151-4.
Morello G, Molino C, Sidoti MT, Parrinello L, Laudani A. Glidescope medium blade vs macintosh blade: laryngoscopy and intubation in 300 patients. Anesthesiology 2009: A475.
Vernick C, Audu P, Mandato P, Heitz J, Bader S. Comparing the Glidescope (GL) with Macintosh laryngoscope (Mac) for intubating difficult airway. Anesthesiology 2006: A534.
Yeatts D, Grissom T, Dutton R, et al. Video laryngoscopy does not improve outcomes in emergency intubation. Crit Care Med 2010: A929.
Shimada M, Hirabayashi Y, Seo N. Nasotracheal intubation using GlideScope videolaryngoscope or Macintosh laryngoscope by novice laryngoscopists (Japanese). Masui 2010; 59: 1318-20.
Mihai R, Blair E, Kay H, Cook TM. A quantitative review and meta-analysis of performance of non-standard laryngoscopes and rigid fibreoptic intubation aids. Anaesthesia 2008; 63: 745-60.
Aziz MF, Healy D, Kheterpal S, Fu RF, Dillman D, Brambrink AM. Routine clinical practice effectiveness of the Glidescope in difficult airway management: an analysis of 2, 004 Glidescope intubations, complications, and failures from two institutions. Anesthesiology 2011; 114: 34-41.
Cooper RM, Pacey JA, Bishop MJ, McCluskey SA. Early clinical experience with a new videolaryngoscope (GlideScope) in 728 patients. Can J Anesth 2005; 52: 191-8.
Hirsch-Allen AJ, Ayas N, Mountain S, Dodek P, Peets A, Griesdale DE. Influence of residency training on multiple attempts at endotracheal intubation. Can J Anesth 2010; 57: 823-9.
Jaber S, Amraoui J, Lefrant JY, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med 2006; 34: 2355-61.
Royal College of Physicians and Surgeons of Canada. Objectives of Training in Internal Medicine, 2003. Available from URL: http://deptmed.queensu.ca/residency/assets/RCPSC_Internal_Medicine_Objectives.pdf (accessed September 2011).
American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 7.1: Adjuncts for airway control and ventilation. Circulation 2005; 112(24_suppl): IV-51-7.
Wayne MA, McDonnell M. Comparison of traditional versus video laryngoscopy in out-of-hospital tracheal intubation. Prehosp Emerg Care 2010; 14: 278-82.
Reade MC, Delaney A, Bailey MJ, Angus DC. Bench-to-bedside review: avoiding pitfalls in critical care meta-analysis-funnel plots, risk estimates, types of heterogeneity, baseline risk and the ecologic fallacy. Crit Care 2008; 12: 220.
Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007; 176: 1091-6.
Acknowledgements
We thank Dr. Seiji Ishikawa for translating the article published in Japanese.
Funding
Dr. Griesdale is supported by a Clinician Scientist Award from the Vancouver Coastal Health Research Institute.
Competing interests
None declared.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Donald E.G. Griesdale was the principle investigator and responsible for the concept and design of the study. He had access to all of the data and takes full responsibility for the integrity of the data and the accuracy of the data analysis. He was also involved in interpretation of the data and drafting of the manuscript. He has no conflicts of interest and approves of the final submitted version of the manuscript. David Liu was involved in the design of the study. He was also involved in acquisition, abstraction, and interpretation of the data. He also helped draft and critically revised the manuscript. He has no conflicts of interest and approves of the final submitted version of the manuscript. James McKinney was involved in the design of the study. He was also involved in acquisition, abstraction, and interpretation of the data. He helped critically revise the manuscript. He has no conflicts of interest and approves of the final submitted version of the manuscript. Peter Choi was involved in the design of the study. He was involved in interpretation of the data and helped draft the manuscript. He also revised the manuscript prior to submission. He has no conflicts of interest and approves of the final submitted version of the manuscript.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Griesdale, D.E.G., Liu, D., McKinney, J. et al. Glidescope® video-laryngoscopy versus direct laryngoscopy for endotracheal intubation: a systematic review and meta-analysis. Can J Anesth/J Can Anesth 59, 41–52 (2012). https://doi.org/10.1007/s12630-011-9620-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-011-9620-5