Abstract
Introduction
Perioperative pain management influences both the quality as well as the speed of recovery following surgery.
Methods
This was a randomized double-blind placebo-controlled study designed to assess the effectiveness of a multimodal analgesic approach (MMA) vs patient-controlled analgesia (PCA) alone in patients undergoing open prostatectomy. Prior to surgery, paravertebral blocks (PVBs) were performed with either 0.5% ropivacaine in the MMA group or saline in the PCA group. Patients in the MMA group also received celecoxib (400 mg po prior to surgery and 200 mg po bid for seven days following surgery) and ketamine 10 mg iv. Following surgery, every patient had free access to morphine PCA. A pain numerical rating scale (NRS) at 24 hr was chosen as the primary endpoint. Secondary endpoints included morphine consumption at 24 hr and SF-36 (36-Item Short-Form Health Survey) scores from two weeks to 24 weeks following surgery.
Results
The primary endpoint, average pain NRS at 24 hr, was 2.6 in the MMA group compared with 3.9 in the PCA group (difference = −1.6, 95% confidence interval [CI]: −2.3 to −0.4; P = 0.01). The average morphine consumption at 24 hr was 4.8 mg in the MMA group compared with 10.5 mg in the PCA group (difference = −5.7, 95% CI: −13.0 to 0.5; P = 0.01). Higher SF-36 scores at two, four, eight, and 12 weeks were observed in the MMA group compared with the PCA group, but no statistically significant (P < 0.05) between-group difference was found after Bonferroni correction of comparisons conducted repeatedly over time. Postoperative adverse effects included low episodes of postoperative nausea and vomiting, bladder spasms, constipation, and pruritus.
Conclusion
This study demonstrates that PVBs combined with celecoxib and ketamine provide better immediate postoperative pain control and facilitate earlier functional recovery in patients undergoing an open radical prostatectomy when compared with PCA alone.
Résumé
Introduction
La prise en charge de la douleur périopératoire influence la qualité aussi bien que la rapidité de rétablissement après une chirurgie.
Méthode
Nous avons réalisé une étude randomisée contrôlée par placebo à double insu afin d’évaluer l’efficacité d’une approche analgésique multimodale (AMM) par rapport à une analgésie contrôlée par le patient (ACP) seule chez des patients devant subir une prostatectomie ouverte. Avant la chirurgie, des blocs paravertébraux (BPV) ont été réalisés à l’aide de 0,5% ropivacaïne dans le groupe AMM ou d’un sérum physiologique dans le groupe ACP. Les patients du groupe AMM ont également reçu du célécoxib (400 mg po avant la chirurgie et 200 mg po bid pendant 7 jours après la chirurgie) et de la kétamine 10 mg iv. Après la chirurgie, une ACP de morphine était à disposition de chaque patient. Le critère d’évaluation principal retenu était une échelle d’évaluation numérique (ÉÉN) à 24 h. Les critères secondaires étaient la consommation de morphine à 24 h et les scores au questionnaire SF-36 (36-Item Short-Form Health Survey – bref questionnaire de santé en 36 éléments) administré entre deux et 24 semaines après la chirurgie.
Résultats
Le critère d’évaluation principal, soit la douleur moyenne sur une ÉÉN à 24 h, était de 2,6 dans le groupe AMM par rapport à 3,9 dans le groupe ACP (différence = −1,6, intervalle de confiance [IC] 95%: −2,3 à −0,4; P = 0,01). La consommation moyenne de morphine à 24 h était de 4,8 mg dans le groupe AMM comparativement à 10,5 mg dans le groupe ACP (différence = −5,7, IC 95%: −13,0 à 0,5; P = 0,01). Des scores plus élevés au SF-36 à deux, quatre, huit et 12 semaines ont pu être observés dans le groupe AMM par rapport au groupe ACP, mais aucune différence intergroupe significative d’un point de vue statistique (P < 0,05) n’a été observée après les corrections des comparaisons selon la méthode Bonferroni réalisées à divers intervalles. Les effets secondaires postopératoires étaient de faibles épisodes de nausées et vomissements postopératoires, des spasmes de la vessie, de la constipation et du prurit.
Conclusion
Cette étude démontre que les BPV combinés au célécoxib et à la kétamine procurent un meilleur soulagement de la douleur postopératoire immédiate et facilitent une récupération fonctionnelle précoce chez les patients subissant une prostatectomie radicale ouverte comparativement à une ACP seule.
Similar content being viewed by others
It is well established that perioperative pain management influences both the quality as well as the speed of recovery following surgery.1,2 Traditionally, treatment of postoperative pain relied primarily on opiates administered either intravenously or epidurally.3,4 Although effective, the administration of opioids is associated with a number of side effects, including nausea and vomiting, ileus, pruritus, sedation, respiratory depression, and immunosuppression, all of which can impair patient recovery.4 Current recommendations for treating postoperative pain recognize the shortcomings of opioids and emphasize the use of multimodal opioid-sparing therapy.5 The evidence suggests that the use of paravertebral blocks provides effective postoperative pain control following breast and thoracic surgery as well as for inguinal hernia repair.6-10 On their own, paravertebral blocks have been demonstrated to provide effective postoperative analgesia lasting up to 24 hr. To complement the benefits of regional analgesia, it has also been established that multimodal analgesic therapy represents a better approach to perioperative pain management than patient-controlled analgesia (PCA) alone in patients undergoing major surgery, including colon resection, nephrectomy, and total hip and knee replacement.11
In a previous study,12 we compared the effects of paravertebral bocks (PVBs) with historical control. We found that patients who received PVBs consumed fewer opioids. Although we could not identify differences between the two patient groups that might explain our observation, we could not rule out the possibility of confounding by an unmeasured variable. To address this potential bias, we conducted a randomized double-blind placebo-controlled study with the goal to compare the effectiveness of a multimodal approach using PVBs with the effectiveness of PCA alone for the management of perioperative pain in patients undergoing radical retropubic prostatectomy. In this trial, we also wanted to assess the long-term benefit of our treatment modality.
Methods
After approval by the institutional review board, patients who were scheduled to undergo elective open radical retropubic prostatectomy for the treatment of prostate cancer by one surgeon (J.B.N.) were screened for enrolment in the study.
Inclusion criteria: 1) male subjects, 2) subjects 35-85 yr-of-age scheduled to undergo open radical retropubic prostatectomy.
Exclusion criteria: 1) gross neurologic impairment, 2) chronic painful conditions, 3) chronic preoperative opioid use, 4) history of coagulation abnormalities, 5) allergy to any medications used in the study protocol, 6) personal or family history of malignant hyperthermia, 7) American Society of Anesthesiologists physical status IV or greater, and 8) any comorbid condition, in the judgement of the urologic surgeon or intraoperative anesthesiologist, that would proscribe the patient from any aspect of the study, such as renal insufficiency (serum creatinine concentration >1.5 g·dL−1), hepatic (serum glutamic oxaloacetic transaminase and serum glutamic pyuvic transaminase 3 X the upper limits of normal) and cardiovascular conditions, including cardiac failure ejection fraction (EF) <30% and uncontrolled and unstable coronary angina.
The patients provided written consent at least two weeks prior to surgery at the time of their visit with the surgeon. In compliance with the Health Insurance Portability and Accountability Act, the surgeon informed the patients of the study, and if they indicated an interest to participate, a research coordinator provided them with an informed consent for their review. On the day of surgery, the patients who chose to participate were asked to confirm their willingness to take part in the study, and those who confirmed their involvement were randomized into one of two groups (multimodal approach group [MMA] or placebo multimodal: control [PCA]). Each patient was asked to complete a SF-36 health survey (a 36-Item Short-Form Health Survey) to assess functional status and was taught the use of the verbal pain numerical rating scale (NRS) and the PCA.
In group MMA, PVBs were performed bilaterally at levels T10, T11, and T12 with 0.5% ropivacaine 5 mL. These patients also received two celecoxib 200 mg tablets 45-120 min prior to surgery and ketamine 10 mg iv (1 mL) following induction of anesthesia. Following surgery, celecoxib 200 mg was administered twice a day for seven days.
In group PCA, paravertebral injections were performed using preservative free 0.9% sodium chloride 5 mL bilaterally at levels T10, T11, and T12. The patients also received two placebo tablets 45-120 min prior to surgery and saline iv (1 mL) following induction of anesthesia. Following surgery, a placebo tablet was administered twice a day for seven days.
To maintain the double-blind study design, neither the patient nor any member of the direct patient care team (physicians, nurses, aides, study monitors) were aware of the arm to which the patient was assigned. An independent pharmacist created a simple computer-generated randomization sequence (1:1)Footnote 1 and used sealed envelopes to conceal identity. To complete the in-patient part of the protocol, the pharmacist dispensed the necessary drugs in a bag labelled according to each patient’s randomization assignment, either active medications (celecoxib, 0.5% ropivacaine in 3-10 mL syringes, and ketamine 1 mL in a 3 mL syringe) or placebo (placebo tablets, saline in 3-10 mL syringes, and saline 1 mL in a 3 mL syringe).
For each component of the treatment, it was impossible to distinguish between the active drug and the placebo.13 Only the pharmacist preparing the study medications knew the patient assignment, and at no time did the pharmacist have direct contact with the patient or any member of the care team. All relevant medications were labelled “study medication” and identified with a patient assignment number as determined by the pharmacist. Prior to discharge from hospital, each patient received either celecoxib or placebo (according to the patient’s randomization number) for an additional seven days of treatment.
Paravertebral block procedure
After an intravenous infusion was established and standard monitors were applied, each patient was positioned in the sitting position prior to being transferred to the operating room. The paravertebral blocks were performed using a 22G Tuohy needle (B-Braun, Bethlehem, PA, USA). The needle was injected once the site of introduction was marked on the patient’s skin (2.5 cm lateral to the spinous processes of levels T10, T11, and T12) and 1% lidocaine was infiltrated subcutaneously.12
Anesthesia
The anesthetic regimen for both groups was identical. After preoxygenation, standard general anesthesia was induced with propofol 2 mg·kg−1, succinylcholine 1 mg·kg−1, and fentanyl 2 μg·kg−1, and the patients’ tracheas were intubated. Paralysis was maintained with rocuronium titrated by twitch monitor to maintain fewer than two twitches of a train-of-four. Isoflurane (0.5-1.5% end-tidal) and fentanyl were used to maintain general anesthesia. Intravenous fluid therapy consisting of lactated Ringer’s solution was administered at the discretion of the anesthesia team and supplemented with 6% Hespan (up to 1,000 mL) and blood products as indicated. At the beginning of surgery (132 ± 51 min after the performance of the paravertebral blocks) following incision of the anterior rectus fascia, the surgeon infiltrated the plane posterior to the fascia but anterior to the rectus muscle with 0.5% ropivacaine 30 mL along the length of the incision. A similar local injection of 0.5% ropivacaine 30 mL was repeated 111 ± 28 min later prior to the surgical closure. At the conclusion of surgery, neuromuscular blockade was reversed and isoflurane was discontinued, and tracheal extubation was performed prior to transferring the patient to the recovery room. Patients in each group received postoperative nausea and vomiting (PONV) prophylaxis prior to the end of surgery.
Postoperative management
In the recovery room, supplemental oxygen was given as needed to maintain oxygen saturation > 94% as measured by pulse oximetry (SpO2). Postoperative nausea and vomiting was treated using ondansatron 4 mg iv followed by promethazine 25 mg iv (if necessary). Morphine 1-2 mg iv was given every ten minutes as needed to control immediate postoperative pain until the patient had free access to a morphine set up delivering a morphine bolus of 1 mg with an eight-minute lockout, no basal infusion, and no limit for the first 24 postoperative hours. A member of the Acute Interventional Perioperative Pain Service (AIPPS) increased the dose of morphine by 50% if the patient’s pain was >5 on a NRS scale of 0-10 (0 = no pain and 10 = the worst possible pain). After 24 hr, the morphine was replaced by acetaminophen with codeine po (300 mg/30 mg) every six hours as needed for pain. Each patient was followed postoperatively by the AIPPS.
Data collection
Pain assessment according to the NRS scale was performed postoperatively every six hours (± two hours) until discharge, although the patients were not wakened for pain assessment during the night. Postoperative morphine consumption was also recorded at 24 hr and 48 hr (morphine equivalent). Postoperatively, the SF-36 Health Survey (0-100) was administered at two, four, eight, 12, and 24 weeks (± three days) by telephone interviews or during routine postoperative office visits.
Statistical analysis
Sample size requirements were based on our findings of an earlier study of pre-emptive analgesia for patients undergoing radical prostatectomy.11 Effect size is defined as the standardized mean difference (e.g., difference in means of two groups/standard deviations [SDs]). Assuming an alpha level of 0.05, a total sample size of 70 patients (35 patients per group) would provide a statistical power of 80% to detect an effect size as small as 0.60 SD units using a one-sided test.
The pain NRS score at 24 hr was chosen as the primary outcome variable. Secondary outcomes included the SF-36 scores from two days to 24 weeks, opioid consumption at 24 hr and 48 hr, respiratory depression (defined as a respiratory rate <eight breaths·min−1 that necessitated treatment with naloxone or mechanical ventilation), frequency of PONV, and perioperative complications, including bladder spasms, myocardial infarction, unexpected need for postoperative intensive care, etc. Data were presented as mean SD for continuous variables normally distributed, as median with interquartile for those continuous variables not normally distributed, or as number (percentage) for episodes of side effects.
All null hypotheses were defined as no difference between group MMA and group PCA and were tested using a two-sided test at the 5% level of significance. The scaled (and/or continuous) endpoints were compared between two groups using a distribution-free rank-sum test, and categorical (nominal) variables were compared using Fisher’s exact test. The Bonferroni procedure was used to adjust P values where between-group comparisons were conducted repeatedly over time. The statistical software, R, (http://www.R-project.org) was used to perform the above analyses.
Results
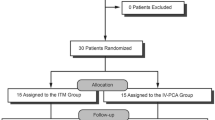
A total of 76 patients signed the informed consent. Fourteen patients withdrew consent prior to the day of surgery, and two were excluded because they did not meet the inclusion criteria. Of the remaining 60 patients who were randomized and medicated, one of the 29 patients in the MMA group and four of the 31 patients in the PCA group withdrew early (Fig. 1). The patients who withdrew from the study did so because they were not satisfied with their postoperative pain management and wanted to receive “active pain medications”. Patient demographics and baseline characteristics were similar across the two groups (Table 1).
Table 2 presents the results of between-group comparisons of the primary and secondary outcomes and adverse effects. The primary outcome, average pain NRS at 24 hr, was 2.6 in the MMA group compared with 3.9 in the PCA group (difference = −1.6, 95% confidence intervals [CI]: −2.3 to −0.4; P = 0.01). The average morphine consumption at 24 hr was 4.8 mg in the MMA group compared with 10.5 mg in the PCA group (difference = −5.7, 95% CI: −13.0 to 0.5, P = 0.01).
Figure 2 presents the median and 25-75th percentile range of the SF-36 scores at two, four, eight, 12, and 24 weeks following surgery for the patients in the two groups. Higher median SF-36 scores were observed in the MMA group compared with the PCA group, but no statistical significance (P < 0.05) was reached after Bonferroni correction of between-group comparisons conducted repeatedly over time.
Plot of the median and 25-75th percentile range postoperative SF-36 scores at two, four, eight, 12, and 24 weeks in patients from the multimodal analgesic approach (MMA) group and the patient-controlled analgesia (PCA) group. The number of patients with complete observations was plotted at each time point under the x-axis. Throughout the study, patients in the MMA group had higher SF-36 scores than patients in the PCA group, although no differences were statistically significant following Bonferroni correction of between-group comparisons conducted repeatedly over time
Adverse events
No serious complications or immediate postoperative infections were observed in either group. On the day of surgery, adverse effect bladder spasms were reported in 46.4% (n = 13) of patients in the MMA group compared with 40.7% (n = 13) of patients in the PCA group, and PONV was reported in 17.9% (n = 5) of patients in the MMA group compared with 18.5% (n = 5) of patients in the PCA group. On postoperative days one and two, the episodes of PONV and bladder spasms were greatly reduced; we also observed fewer of these episodes and less constipation in the MMA group than in the PCA group, although none of the differences were statistically significant (Table 2).
Discussion
This study demonstrates that a multimodal approach, including paravertebral blocks, celecoxib, and ketamine, provides better postoperative pain control than PCA morphine alone in patients undergoing open radical retropubic prostatectomy. This approach also allows a reduction in the postoperative need for opioids, lessens the related side effects (e.g., PONV, constipation, and bladder spasm), and facilitates earlier patient recovery.
This study was not designed to assess the effects of paravertebral block alone, as this approach would have required a control group to receive ketamine and celecoxib as well. The study was designed to compare our multimodal approach with PCA, which remains the gold standard for the management of perioperative pain. An additional study would be required to assess the respective roles played by each component of this multimodal approach. Such a study would entail a 12-group factorial design and enrolment of several hundred patients,14 which could be achieved only by using a multicentre approach.
Epidural analgesia has been considered the regional technique of choice for patients undergoing open radical prostatectomy.15 Our data indirectly suggest that paravertebral blocks represent an interesting alternative to the use of epidural analgesia in this surgical population. Direct comparisons between paravertebral blocks and epidural analgesia have shown that paravertebral blocks produce similar analgesia and have the advantage of being associated with fewer risks of major hypotension, urinary retention, and PONV.16,17 Another advantage of regional anesthesia is the possibility that its use may delay the development of cancer. This concept has been demonstrated in animals,18 but the idea was later suggested in humans using historical data.19,20 However, data from prospective studies are required to confirm this concept.
Several multimodal approaches have been advocated based on different combinations of anti-inflammatory drugs, including COX2 inhibitors (rofecoxib, valdecoxib, and celecoxib), acetaminophen, N-methyl-D-aspartate (NMDA) inhibitors (ketamine), gabapentin or pregabalin, steroids, and regional anesthesia (epidural, peripheral nerve blocks, paravertebral blocks, and local injection/infusion of local anesthetics).21-24 Although each of these drugs and/or techniques has been demonstrated as being effective in reducing the need for postoperative intravenous opioids alone, the evidence supporting specific combinations of drugs and/or regional techniques is still limited. In this respect, our study demonstrated that the following approach, i.e., administering COX2 inhibitor (celecoxib) pre and post surgery, giving a single intravenous dose of an NMDA inhibitor (ketamine) immediately prior to surgery, and performing bilateral paravertebral blocks at levels T10, T11, and T12 prior to surgery, represents an effective alternative to the multimodal protocols previously proposed.11
The design of this study has its limitations. The study did not include an assessment of the relative role played by each component of our multimodal perioperative pain protocol (celecoxib vs ketamine vs paravertebral block vs local infiltration). The multimodal approach to pain management developed for this patient population was based on our prior experience using a similar approach in orthopedics (regional technique combined with the use of a COX2 inhibitor and a NMDA inhibitor). Determining the relative role played by each component of the multimodal approach would require a “metric” design and would involve enrolling a much larger number of patients, as is the case for PONV trials. Therefore, within the context of a regional-based multimodal approach to perioperative pain management, additional studies are required to establish further the relative role of ketamine, celecoxib, and even the local infiltration.
Our study demonstrates that a multimodal approach incorporating paravertebral blocks represents a more effective alternative than morphine PCA for the perioperative pain management of patients undergoing open radical prostatectomy. Our data also support the concept that such an approach facilitates the patient’s early recovery.
Notes
Randomization plans. Available from http://www.tufts.edu/~gdallal/permute.htm (accessed November 2010).
References
Carr DB, Goudas LC. Acute pain. Lancet 1999; 353: 2051-8.
Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA 1998; 279: 847-52.
Kissin I. Patient-controlled-analgesia analgesimetry and its problems. Anesth Analg 2009; 108: 1945-9.
Gottschalk A, Smith DS, Jobes DR, et al. Preemptive epidural analgesia and recovery from radical prostatectomy: a randomized controlled trial. JAMA 1998; 279: 1076-82.
American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2004; 100: 1573-81.
Pusch F, Freitag H, Weinstabl C, Obwegeser R, Huber E, Wildling E. Single-injection paravertebral block compared to general anesthesia in breast surgery. Acta Anaesthesiol Scand 1999; 43: 770-4.
Klein SM, Greengrass RA, Weltz C, Warner DS. Paravertebral somatic nerve block for outpatient inguinal herniorrhaphy: an expanded case report of 22 patients. Reg Anesth Pain Med 1998; 23: 306-10.
Greengrass R, O’Brien F, Lyerly K, et al. Paravertebral block for breast cancer surgery. Can J Anaesth 1996; 43: 858-61.
Greengrass R, Buckenmaier CC 3rd. Paravertebral anesthesia/analgesia for ambulatory surgery. Best Pract Res Clin Anaesthesiol 2002; 16: 271-83.
Karmakar MK. Thoracic paravertebral block. Anesthesiology 2001; 95: 771-80.
Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008; 248: 189-98.
Ben-David B, Swanson J, Nelson JB, Chelly JE. Multimodal analgesia for radical prostatectomy provides better analgesia and shortens hospital stay. J Clin Anesth 2007; 19: 264-8.
Moher D, Hopewell S, Schulz KF, Consolidated Standards of Reporting Trials Group, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010; 63: e1-37.
Apfel CC, Korttila K, Abdalla M, IMPACT Investigators, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 2004; 350: 2441-51.
Gupta A, Fant F, Axelsson K, et al. Postoperative analgesia after radical retropubic prostatectomy: a double-blind comparison between low thoracic epidural and patient-controlled intravenous analgesia. Anesthesiology 2006; 105: 784-93.
Richardson J, Sabanathan S, Jones J, Shah RD, Cheema S, Mearns AJ. A prospective, randomized comparison of preoperative and continuous balanced epidural or paravertebral bupivacaine on post-thoracotomy pain, pulmonary function and stress responses. Br J Anaesth 1999; 83: 387-92.
Mohta M, Verma P, Saxena AKr, Sethi AK, Tyagi A, Girotr G. Prospective, randomized comparison of continuous thoracic epidural and thoracic paravertebral infusion in patients with unilateral multiple fractured ribs—a pilot study. J Trauma 2009; 66: 1096-101.
Wada H, Seki S, Takahashi T, et al. Combined spinal and general anesthesia attenuates liver metastasis by preserving Th1/Th2 cytokine balance. Anesthesiology 2007; 106: 499-506.
Biki B, Mascha Ed, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology 2008; 109: 180-7.
Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology 2006; 105: 660-4.
Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg 2010; 110: 199-207.
Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg 2005; 101: 1427-32.
Clarke H, Pereira S, Kennedy D, et al. Adding gabapentin to a multimodal regimen does not reduce acute pain, opioid consumption or chronic pain after total hip arthroplasty. Acta Anaesthesiol Scand 2009; 53: 1073-83.
Mathiesen O, Rasmussen ML, Dierking G, et al. Pregabalin and dexamethasone in combination with paracetamol for postoperative pain control after abdominal hysterectomy. A randomized clinical trial. Acta Anaesthesiol Scand 2009; 53: 227-35.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chelly, J.E., Ploskanych, T., Dai, F. et al. Multimodal analgesic approach incorporating paravertebral blocks for open radical retropubic prostatectomy: a randomized double-blind placebo-controlled study. Can J Anesth/J Can Anesth 58, 371–378 (2011). https://doi.org/10.1007/s12630-010-9442-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-010-9442-x