Abstract
Obesity is a public health problem characterized by increased accumulation of fat into adipose tissues leading to oxidative stress, dyslipidemia, and chronic inflammatory status. We used an experimental model of high-fat diet-induced obesity to analyze the link between dyslipidemia, oxidative stress, and fat accumulation into adipose tissue of rats, as well as the involvement of intracellular mediators such as transition metals on signal transduction. We also looked at the ability of a grape seed and skin extract (GSSE) from a Tunisian cultivar to prevent fat-induced disturbances. Data showed that a high-fat diet (HFD) provoked dyslipidemia into plasma which is linked to an oxidative stress, an accumulation of transition metals such as manganese, copper, and zinc and a depletion of iron. GSSE prevented dyslipidemia by modulating lipase activity, together with increased antioxidant capacity and depletion of transition metals as well as of free radicals such as O2 − and OH. These data indicated that GSSE has important preventive effects on HFD-induced obesity and oxidative stress whose transduction seems to involve transition metals. GSSE should be used as a safe anti-obesity agent that could find potential applications in metabolic disorders involving transition metals dyshomeostasis.
Similar content being viewed by others
Introduction
Obesity is a public health concern characterized by excessive fat deposition into adipocytes and non-adipose tissues, which is accompanied by a cluster of chronic metabolic disorders including cardiovascular diseases, type 2 diabetes, steatohepatitis, and dyslipidemia. Obesity and metabolic disorders are also linked to an overt oxidative stress and chronic inflammatory status. Oxidative stress along with a decline in antioxidant defenses cause an irreversible damage to macromolecules [1] and a disruption in redox signaling mechanisms [2]. A growing number of studies suggest a potential link between obesity and altered transition metals metabolism. For instance,an association between iron deficiency and obesity has long been recognized [3], as well as between plasma copper levels and inflammatory status-induced metabolic disorders [4]. Moreover, higher plasma levels of copper, iron, and manganese were recently described in obese rats when compared to their lean counterpart [5].
Since current medical treatments fail to stop the progress of metabolic disorders, polyphenol-rich grape products are being widely investigated as an additional strategy to combat obesity [6]. Grape seed and skin extract (GSSE) is a complex mixture of bioactive compounds including polyphenolics, flavonoids, proanthocyanidins, and unsaturated fatty acids which has been recently attributed the Generally Recognized as Safe (GRAS) certification from the US FDA [7]. GSSE exerts numerous biological activities and health-promoting properties such as antioxidant [8], lipid lowering [9], anti-tumor [10], and anti-obesity effects [11] by inhibiting lipid absorption from the intestine which has been shown to occur partly via inhibition of lipases [12].
In the present study, we investigated the effect of a high-fat diet on plasma and white adipose tissue lipid deposition and oxidative stress in rat. We also analyzed the protection offered by GSSE with a special emphasis on plasma biomarkers as free radicals and transition metals.
Materials and methods
Reagents and diets
Grape seed and skin extract was processed from a grape cultivar (Carignan) of Vitis vinifera from northern Tunisia. Seeds were manually separated from skin, air-dried, and ground separately with a coffee grinder (FP 3121 Moulinex) until a fine powder was obtained. Both powders were then mixed at a 50:50 ratio on a dry weight basis in 10 % ethanol (v/v) in the dark. After vigorous stirring and centrifugation at 10,000g for 15 min at 4 °C, the supernatant containing soluble polyphenols was administered by intraperitoneal (i.p.) route to animals. Total phenolic content and GSSE composition (Table 1) were determined according to [13]. Standard diet (SD) for rodent in pelleted form was purchased from ALMAS Bizerta (Tunisia) and contained 3 % fat, 40 % carbohydrate, and 14.5 % protein. High-fat diet (HFD) was prepared by soaking commercial food pellets into warmed (100 °C) and liquefied fat (peri-renal) from animal origin (sheep) during 15 min and allowed to dry at room temperature. HFD contained 28 % fat, 32 % carbohydrate, and 11.6 % protein. Composition of SD and HFD is shown in Table 2 and is mainly characterized by less carbohydrate (40 %) than the AIN-93 M diet (67.5 %).
Animals and experimental design
Twenty-four male Wistar rats (210–230 g) from the Pasteur Institute (Tunis) were used in agreement with the NIH guidelines [14]. They were maintained in the animal facility at a controlled temperature (22 ± 2 °C), a 12-h light/dark cycle, and divided into 4 groups of 6 animals each fed either SD or HFD for 6 weeks. Rats daily received by i.p. injection either 10 % ethanol as vehicle (control SD and HFD) or various doses of GSSE from 250 to 4,000 mg/kg, on the basis of the weight of the starting dry material. At the end of the treatment, rats were killed by decapitation, their blood collected, and the plasma processed for lipid profile and oxidative stress assessment.
Plasma biochemical analysis
LDL-cholesterol (LDL-C) was determined using a commercial kit from Biolabo (France) and HDL-cholesterol (HDL-C) using a kit from Biomaghreb, (Tunisia). HDL-Phospholipid (HDL-PL) and (LDL + VLDL)-Phospholipid (LDL + VLDL)-PL were determined using phospholipid assay kits purchased from BioMérieux,Marcy-l’etoile, (France). C-reactive protein (CRP), an inflammatory biomarker, was measured using a konelab clinical chemistry analyzer (Thermoclinical Labsystems, Espoo, Finland). Total proteins were determined according to Hartree [15] with bovine serum albumin (BSA) as standard. Plasma lipoperoxidation was evaluated by malondialdehyde (MDA) measurement [16]. Briefly, plasma was precipitated with trichloroacetic acid and MDA from the supernatant was allowed to react with thiobarbituric acid (TBA). Spectrophotometric measurements were conducted at 532 nm and the MDA concentration calculated using the absorbance coefficient of the MDA–TBA complex, 1.56 × 105 cm−1 M−1. Oxidative damage to proteins was evaluated by quantifying protein carbonylation according to Levine et al. [17]. Briefly, after protein precipitation with 20 % TCA and dissolving in 2,4,dinitrophenylhydrazine (DNPH)-containing buffer, absorbance was measured at 366 nm and the results expressed as nmol carbonyl/mg protein.
Reduced (GSH) and oxidized-glutathione (GSSG) were determined according to [18]. Non-protein thiol radicals (NPSH) were determined spectrophotometrically according to [19].
Plasma was also used for antioxidant enzyme activities such as glutathione peroxidase (GPx; E.C.1.11.1.9.) [20], catalase (CAT; E.C.1.11.1.6.) [21], and superoxide dismutase (SOD; E.C.1.15.1.1.) [22]. Characterization of SOD isoforms was performed using KCN (3 mM) as Cu/Zn-SOD inhibitor or H2O2 (3 mM) which affect both Cu/Zn and Fe-SOD, whereas Mn-SOD is insensitive to both inhibitors.
Plasma superoxide anion (O2 −) and hydroxyl radical (OH·) were determined according to the method of Marklund and Marklund [23] and Payà et al. [24], respectively.
Ionizable calcium and H2O2 were measured using commercial kits from Biomaghreb according to Stern and Lewis [25] and Kakinuma et al. [26], respectively. Nitric oxide (NO) levels were determined by quantification of NO metabolites nitrite and nitrate according to Green et al. [27]. Free iron was measured by the ferrozine method according to [28]. Plasma was diluted in nitric acid (15.5 mol/L), and filtered for zinc (Zn), copper (Cu), and manganese (Mn) determinations by atomic absorption spectroscopy.
Adipose tissue analysis
Adipose tissues from abdominal region such as epididymal (EAT), peri-renal (PAT), retroperitoneal (RAT), amd mesenteric (MAT) were carefully dissected and weighed. The absolute weight was measured using a Sartorius balance (Sartorius, Gottingen, Germany), and the relative adipose tissue weight per 100 g body weight was calculated:
Total lipids from adipose tissue were extracted according to Folch et al. [29] and the triglyceride determined enzymatically using a commercial kit from Biomaghreb according to [30]. Adipose tissue lipase activity was determined according to the method of Humbert et al. [31]. A 100-mM solution of p-nitrophenol dodecanoate in dimethylsulfoxide (DMSO) and ethanol was prepared. The reaction mixture contained 5 mM p-nitrophenol dodecanoate, 50 mM Tris–HCl buffer pH 8.5, and 50 μL of sample and was incubated at 37 °C for 1 h after which the reaction was stopped with 60 mM EDTA. After centrifugation at 10,000g for 5 min, absorbance was measured at 412 nm. One unit was defined as the amount of enzyme catalyzing the release of 1 μmol p-nitrophenol (ε = 18.3 mM−1 cm−1)
Statistical analysis
Results are expressed as the mean ± SEM. Data were compared by two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. A P value less than 0.05 was considered significant.
Results
Anthropometric and adipose tissue lipid parameters
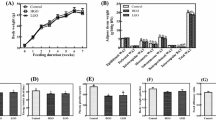
Figure 1a shows that GSSE exerted a dose–response inhibitory effect on body weight which reached a significant level at 500 mg/kg bw. This dose was used throughout the study. We report in Fig. 1b the relative visceral adipose tissue weight changes in animals fed SD or HFD and treated or not with GSSE. The data clearly show that HFD increased EAT by 100 %, MAT by 90 %, PAT by 134 %, and, to a lesser extent, RAT by 8 %, and that GSSE counteracted all HFD-induced fat deposition to near control level. HFD also provoked triglyceride accumulation (Fig. 1c) and reduced lipase activity (Fig. 1d) into abdominal adipose tissue. All these disturbances were restored upon GSSE treatment to near control level.
Protective effect of GSSE on HFD-induced body weight (a), EAT, MAT, RAT, and PAT weights (b), adipose tissue, triglyceride (c) and adipose tissue lipase activity (d) changes. Rats were fed a SD or HFD during 6 weeks and daily injected either with a fixed dose (500 mg/kg bw) or with various doses of GSSE. Results are expressed as mean ± SEM. p < 0.05 was considered significant. #for SD + GSSE vs. SD, *for HFD vs. SD, and §for HFD + GSSE vs. HFD
Adipose tissue oxidative stress
The high-fat diet induced an oxidative stress into adipose tissue characterized by an increase in MDA by 74 % (Fig. 2a) but not carbonylation (Fig. 2b), and by an increase in free iron by 64 % (Fig. 2c) and H2O2 by 38 % (Fig. 2d). It is noteworthy that GSSE, which exerted antioxidant properties in basal SD conditions, counteracted almost all HFD effects to near control level. Furthermore, HFD affected antioxidant enzyme activities such as GPx by −67 % (Fig. 3a) and SOD by −31 % (Fig. 3b) but had no effect on CAT (Fig. 3c). GSSE partially restored antioxidant enzyme activities to near control level.
Protective effect of GSSE on HFD-induced MDA (a), carbonyl protein (b), free iron (c), and H2O2 (d) changes in adipose tissue. Rats were fed a SD or HFD during 6 weeks and daily administered with GSSE (500 mg/kg bw). Results are expressed as mean ± SEM. p < 0.05 was considered significant: *for HFD vs. SD, and §for HFD + GSSE vs. HFD
Protective effect of GSSE on HFD-induced GPx (a), SOD (b), and CAT (c) activity changes in adipose tissue. Rats were fed a SD or HFD during 6 weeks and daily administered with GSSE (500 mg/kg bw). Results are expressed as mean ± SEM. p < 0.05 was considered significant: *for HFD vs. SD, and §for HFD + GSSE vs. HFD
Plasma lipid
We report in Fig. 4 the plasma level of various lipid species from SD or HFD fed animals. HFD highly increased LDL-C/HDL-C by 137 % (Fig. 4a) and (LDL + VLDL)-PL/HDL-PL ratios by 159 % (Fig. 4b) and decreased HDL-C by 39 % as well as HDL-PL by 5 %. GSSE reversed all these HFD-induced disturbances to control level.
Protective effect of GSSE on HFD-induced plasma LDL-C/HDL-C (a) and (LDL + VLDL)-PL/HDL-PL (b) ratios. Rats were fed a SD or HFD during 6 weeks and daily treated with GSSE (500 mg/kg bw). Results are expressed as mean ± SEM. p < 0.05 was considered significant: *for HFD vs. SD, for HFD + GSSE vs. HFD
Plasma oxidative stress
We further sought to determine whether HFD induced an oxidative stress within plasma (Fig. 5). HFD slightly increased MDA by 28 % (Fig. 5a) and, more extensively, carbonyl protein by 25 % (Fig. 5b). HFD also decreased the reducing power as assessed by sulfhydryl radicals by 68 % (Fig. 5c) as well as glutathione and GSH/GSSG ratio by 36 % (Fig. 5d). GSSE treatment brought all these parameters to near control level.
Protective effect of GSSE on HFD-induced plasma lipoperoxidation (a), carbonyl protein (b), sulfhydryl radicals (c) and glutathione (d) changes. Rats were fed a SD or HFD during 6 weeks and daily administered with GSSE (500 mg/kg bw). Results are expressed as mean ± SEM. p < 0.05 was considered significant: *for HFD vs. SD, and §for HFD + GSSE vs. HFD
HFD treatment also depressed plasma antioxidant enzyme activities such as GPx by 25 % (Fig. 6a), and, to a lesser extent, SOD by 17 % (Fig. 6b), but had no effect on CAT (Fig. 6c). Among SODs, the Cu/Zn isoform was the most affected one (Fig. 6c). GSSE alleviated all the deleterious effects of HFD on antioxidant enzyme activities.
Plasma transition metals
HFD treatment highly elevated plasma Mn by 611 % (Fig. 7a), Zn by 133 % (Fig. 7b), and Cu by 733 % (Fig. 7c), and decreased free iron by 15 % (Fig. 7d). GSSE (500 mg/kg) efficiently abrogated all these disturbances to near control level and dose-dependently in the case of free iron.
Protective effect of GSSE on HFD-induced modulation of plasma Mn (a), Zn (b), Cu (c), and free iron (d). Rats were fed a SD or HFD during 6 weeks and daily injected either with a fixed dose (500 mg/kg bw) or with various doses of GSSE. Results are expressed as mean ± SEM. p < 0.05 was considered significant: *for HFD vs. SD, and §for HFD + GSSE vs. HFD
Plasma CRP and free radicals
We report in Table 3 the effect of HFD on plasma H2O2, NO, O2 −, OH·, and the inflammatory biomarker CRP. Data clearly show that HFD greatly increased the O2 − by 66 %, the OH· by 63 %, and CRP by 380 % with no significant effect on all other parameters. GSSE depressed O2 −, OH·, and CRP in SD condition and abrogated all HFD-induced disturbances to near control level.
Discussion
The present study reported the effect of HFD-induced dyslipidemia and oxidative stress into the plasma and white adipose tissue of rat. HFD induced an overt obesity characterized by body weight gain, and abdominal fat deposition into epididymal, perirenal, mesenteric, and retroperitoneal white adipose tissues. HFD-induced dyslipidemia into plasma was assessed by elevated cholesterol (high LDL and low HDL) and phospholipid (high LDL + VLDL and low HDL) and into adipose tissue by high triglyceride deposition.
HFD also provoked a clear oxidative stress status within plasma, characterized by ROS accumulation, an increase in both lipoperoxidation and protein carbonylation, and a decrease in thiol radicals and glutathione. Consequently, our data further confirmed that obesity is strongly associated with systemic oxidative stress [32] in particular concerning the relationship between altered glutathione homeostasis and organ lipotoxicity associated with diet-induced obesity [33].
HFD treatment also affected GPx and SOD activity, without affecting CAT. This last result is in accordance with the lack of any effect on plasma H2O2, whereas the inhibition of GPx activity is in accordance with the HFD-induced glutathione depletion. The reduced circulating GPx activity is noticeable, associated with the obesity-related rise in systemic oxidative stress and incidence of metabolic complications [34]. Among SODs, the Cu/Zn isoform, which corresponds to the secreted form of the enzyme, was the most affected isoform, which is in phase with the higher O2 − level found into the plasma of HFD-fed animals. These data prompted us to evaluate the effect of HFD on the distribution of transition metals. We found that HFD induced the accumulation of Mn, Cu, and Zn and the depletion of iron. Moreover, HFD increased the catalytic free iron level into adipose tissue and concomitantly reduced the adipocyte lipase activity which is in accordance with triglyceride accumulation and the increase in adipose tissue weight. Our data are also in accordance with those of [35] who highligted the relationship between iron status and lipid metabolism, in particular the ability of free iron to inhibit lipoprotein lipase activity and consequently hypertriglyceridemia. Overall, our data emphasized the usefulness of such an experimental model in studying the relationship between obesity and trace metals homeostasis. We also found that HFD induced the specific accumulation of free iron into the white adipose tissue as previously reported for the heart [36]. Moreover, HFD provoked the depletion of Mn from the brain [13], of Zn from the liver and pancreas (not shown), and of Cu from the heart and kidney [37]. Such a relationship between obesity and transition metals distribution was mentioned several decades ago by Kennedy et al. [38], who described lower levels of micronutrients in tissues and higher levels in plasma from genetically obese mice when compared to lean controls. Serum copper levels were independently associated with dyslipidemia and inflammation. Thus, the role of copper deficiency in lipid metabolism defects such as hypercholesterolemia has long been recognized [39]. High plasma copper was positively correlated with the consumption of monounsaturated and polyunsaturated fatty acids, whereas high plasma manganese was correlated with dairy products consumption [40]. More recently, elevated circulating levels of copper were linked to concentric left ventricular hypertrophy in elderly patients, a defect which is likely inherent to obesity [41]. Manganese over-exposure of rats during 6 weeks induced disturbances in brain lipid metabolism as assessed by high level of palmitate, oleate, and cholesterol [42]. Zinc plays a critical role in the regulation of hepatic lipid metabolism, as dietary zinc supplementation has been shown to reduce alcohol-induced liver steatosis [43]. A growing number of studies suggest a potential link between obesity and altered iron metabolism. For instance, reduction of iron levels by the iron chelator deferoxamine ameliorated obesity through decreased oxidative stress and adipocyte hypertrophy [44], and conversely lipocalin 2 induced cardiomyocyte apoptosis by increasing iron deposition [45]. Future studies should investigate which free fatty acid among those present at high level in our HFD, i.e. palmitate, stearate, and oleate, is specifically linked to transition metals dyshomeostasis. In this respect, HFD-induced obesity was recently shown to up-regulate the abundance of GPR 43 into the liver and GPR120 into the heart [46], which supporta a putative role of these receptors in selectively mediating metabolic processes in these tissues. In addition, palmitate has been previously shown to enhance oxidative stress, apoptosis, and cardiotoxicity, and to increase iron uptake [47].
The powerful ability of GSSE to counteract most of the HFD-induced disturbances such as weight gain, adipose tissue hypertrophy, oxidative stress within adipocytes, and plasma is very interesting. Polyphenolics present in GSSE exerted real antioxidative properties which are in line with several previous reports in the field [48] especially those dealing with the anti-obesity effect of GSSE [11, 49]. GSSE acted as a lipolytic agent by enhancing the lipase activity in white adipose tissue of SD animals and also by counteracting the HFD-induced reduction of lipase activity in fat tissue. It is well recognized that obesity, which is caused by excess caloric intake, can be improved by inhibiting digestive lipase which delays lipid absorption [50]. Natural inhibitors isolated from plants, fungi, algae, or bacteria have been screened for their potential preventive effect on obesity and inhibition of lipase activity, among which are grape polyphenols (reviewed in [6]).
Our data are fully in line with those of [51] who showed the beneficial antiobesity effect of low doses of grape seed procyanidins which increased lipase activity in white adipose tissue in hamsters. However, they are partially in line with those of Moreno et al. [12], who demonstrated in vitro an inhibitory effect of grape seed polyphenols on lipases. We have not yet conducted such in vitro experiments to demonstrate a direct inhibition of adipose tissue lipase by our polyphenol mixture, nor do we know the hypothetical level of lipase regulation (transcriptional or translational) that occurs in vivo, nor which kind of polyphenol is specifically involved in such regulation. In this respect, quercetin could be a good candidate as it has been shown to increase energy expenditure in mice fed a high-fat diet [52], or resveratrol which improved health and survival of mice on a high caloric diet [53]. However, we should keep in mind that the overall effect likely results from synergism between the various GSSE-containing polyphenols.
Another interesting novelty of GSSE is its powerful ability in counteracting HFD-induced disturbances in trace metals distribution into plasma and free iron deposition in adipose tissues. Only a few studies have examined this aspect and part of our data are consistent with the recent work of [5], who underlined a novel role for adipose tissue in iron homeostasis. Of particular interest is a recent work conducted on Hep G2 cells, where GSSE was shown to act by modulating zinc homeostasis by extracellular complexation of zinc and elevation of cytoplasmic labile zinc. Although this effect of GSSE was obtained in vitro, it could be a relevant mechanism underlying the modulation of diverse cell signaling and the metabolic pathway [54].
GSSE is safe and devoid of any pro-oxidant effect up to 4 g/kg which corresponds to 280 g/day for a human adult. Of note in our present experiments, only the soluble polyphenolic fraction of GSSE was administered to the animals, which corresponded to 1 % of the starting powder material and equivalent to 40 mg polyphenol/kg. We are currently testing capsules of GSSE containing 0.5 mg polyphenol/kg which exhibit interesting antioxidant properties in humans in 6-month-long experiments. We also plan the use of more concentrated capsules of 40 mg polyphenol/kg and even more in clinical trials against obesity. Interestingly, such high-dosage capsules constitute a far less invasive alternative than lipectomy [55] or whole body cryostimulation [56] in the treatment of obesity or obesity-associated low grade inflammation, and are also less invasive than phlebotomy in the treatment of metabolic syndrome [57].
Conclusion
Grape seed and skin extract is safe even at very high dosage and should be further tested as a lipolytic and anti-obesity agent. GSSE also exhibited a robust free radical scavenger activity in basal condition (SD) and in HFD-induced oxidative stress. These data open the way to clinical trials using high-dosage polyphenols in the treatment of obesity.
Abbreviations
- EAT:
-
Epididymal adipose tissue
- GSSE:
-
Grape seed and skin extract
- HFD:
-
High-fat diet
- MAT:
-
Mesenteric adipose tissue
- PAT:
-
Perirenal adipose tissue
- RAT:
-
Retroperitoneal adipose tissue
References
Levine RL, Stadtman ER (2001) Oxidative modification of proteins during aging. Exp Gerontol 36:1495–1502
Kamata H, Hirata H (1999) Redox regulation of cellular signalling. Cell Signal 11:1–14
Cheng HL, Bryant C, Cook R, O’Connor H, Rooney K, Steinbeck K (2012) The relationship between obesity and hypoferraemia in adults: a systematic review. Obes Rev 13:150–161
de Luis DA, Pacheco D, Izaola O, Terroba MC, Cuellar L, Martin T (2011) Zinc and copper serum levels of morbidly obese patients before and after biliopancreatic diversion: 4 years of follow-up. J Gastrointest Surg 15:2178–2181
Thompson R (2011) Obesity: novel role for adipose tissue in iron homeostasis. Nat Rev Gastroenterol Hepatol. 8:243
Chuang CC, McIntosh MK (2011) Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu Rev Nutr 31:155–176
FDA agency response letter GRAS notice no. GRN000124. http://www.fda.gov/Food/Foodingredients/Packaging/GenerallyRecognizedasSafeGRAS/GRASListings/ucm/153940.htm. Accessed June 1 2011
Belviranli M, Gökbel H, Okudan N, Büyükbaş S (2012) Oxidative stress and anti-oxidant status in diabetic rat liver: effect of plant polyphenols. Arch Physiol Biochem 118:237–243
Quesada H, Díaz S, Pajuelo D, Fernández-Iglesias A, Garcia-Vallvé S, Pujadas G, Salvadó MJ, Arola L, Bladé C (2012) The lipid-lowering effect of dietary proanthocyanidins in rats involves both chylomicron-rich and VLDL-rich fractions. Br J Nutr 108:208–217
Nandakumar V, Singh T, Katiyar SK (2008) Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett 269:378–387
Ohyama K, Furuta C, Nogusa Y, Nomura K, Miwa T, Suzuki K (2011) Catechin-rich grape seed extract supplementation attenuates diet-induced obesity in C57BL/6J mice. Ann Nutr Metab 58:250–258
Moreno DA, Ilic N, Poulev A, Brasaemle DL, Fried SK, Raskin I (2003) Inhibitory effects of grape seed extract on lipases. Nutrition 19:876–879
Charradi K, Elkahoui S, Karkouch I, Limam F, Ben Hassine F, Aouani E (2012) Grape seed and skin extract prevents high-fat diet-induced brain lipotoxicity in rat. Neurochem Res 37:2004–2013
National Research Council (1985) Guide for the care and the use of laboratory animals, vol 20. National Institute of Health, Bethesda, pp 85–123
Hartree EF (1972) Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 48:422–427
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Levine RL, Garland D, Olover CN, Amici A, Climent I, Lenz AG (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:467–478
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522
Ellman G (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Nakamura K, Hosada S, Hayashi K (1974) Purification and properties of rat liver glutathione peroxidase. Biochem Biophys Acta 358:251–261
Aebi H (1974) Methods of enzymatic analysis, 2nd edn. Chemia Weinheium, New York
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Payá M, Halliwell B, Hoult JR (1992) Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem Pharmacol 44:205–214
Stern J, Lewis WH (1957) The colorimetric estimation of calcium in serum with ocresolphthalein complexone. Clin Chim Acta 2:576–580
Kakinuma K, Yamaguchi T, Kaneda M, Shimada K, Tomita Y, Chance B (1979) Determination of H2O2 release by the treatment of human blood polymorphonuclear leucocytes with myristate. J Biochem 86:87–95
Green LC, Wagner DA, Glogowski J, Shipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal Biochem 126:131–138
Leardi A, Caraglia M, Selleri C, Pepe S, Pizzi C, Notaro R (1998) Desferioxamine increases iron depletion and apoptosis induced by ara-C of human myeloid leukaemic cells. Br J Haematol 102:746–752
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28:2077–2080
Humbert G, Guingamp MF, Linden G (1997) Method for the measurement of lipase activity in milk. J Dairy Res 64:465–469
Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ; Framingham Study (2003) Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 23:434–439
Ghosh S, Sulistyoningrum DC, Glier MB, Verchere CB, Devlin AM (2011) Altered glutathione homeostasis in heart augments cardiac lipotoxicity associated with diet-induced obesity in mice. J Biol Chem 286:42483–442493
Lee YS, Kim AY, Choi JW, Kim M, Yasue S, Son HJ, Masuzaki H, Park KS, Kim JB (2008) Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol Endocrinol 22:2176–2189
Kim J, Jia X, Buckett PD, Liu S, Lee CH, Wessling-Resnick M (2013) Iron loading impairs lipoprotein lipase activity and promotes hypertriglyceridemia. FASEB J 27:1657–1663
Charradi K, Sebai H, Elkahoui S, Ben Hassine F, Limam F, Aouani E (2011) Grape seed extract alleviates high-fat diet-induced obesity and heart dysfunction by preventing cardiac siderosis. Cardiovasc Toxicol 11:28–37
Charradi K, Elkahoui S, Karkouch I, Limam F, Hamdaoui G, Hassine FB, El May MV, El May A, Aouani E (2013) Grape seed and skin extract alleviates high-fat diet-induced renal lipotoxicity and prevents copper depletion in rat. Appl Physiol Nutr Metab 38:259–267
Kennedy ML, Failla ML, Smith JC Jr (1986) Influence of genetic obesity on tissue concentrations of zinc, copper, manganese and iron in mice. J Nutr 116:1432–1441
Kim S, Chao PY, Allen KG (1992) Inhibition of elevated hepatic glutathione abolishes copper deficiency cholesterolemia. FASEB J 6:2467–2471
Sánchez C, López-Jurado M, Aranda P, Llopis J (2010) Plasma levels of copper, manganese and selenium in an adult population in southern Spain: influence of age, obesity and lifestyle factors. Sci Total Environ 408:1014–1020
Lind PM, Olsén L, Lind L (2012) Elevated circulating levels of copper and nickel are found in elderly subjects with left ventricular hypertrophy. Ecotoxicol Environ Saf 86:66–72
Fordahl S, Cooney P, Qiu Y, Xie G, Jia W, Erikson KM (2012) Waterborne manganese exposure alters plasma, brain, and liver metabolites accompanied by changes in stereotypic behaviors. Neurotoxicol Teratol 34:27–36
Sasaki S, Zhang X, Kesteloot H (1995) Dietary sodium, potassium, saturated fat, alcohol, and stroke mortality. Stroke 26:783–789
Tajima S, Ikeda Y, Sawada K, Yamano N, Horinouchi Y, Kihira Y, Ishizawa K, Izawa-Ishizawa Y, Kawazoe K, Tomita S, Minakuchi K, Tsuchiya K, Tamaki T (2012) Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. Am J Physiol Endocrinol Metab 302:77–86
Xu G, Ahn J, Chang S, Eguchi M, Ogier A, Han S, Park Y, Shim C, Jang Y, Yang B, Xu A, Wang Y, Sweeney G (2012) Lipocalin-2 induces cardiomyocyte apoptosis by increasing intracellular iron accumulation. J Biol Chem 287:4808–4817
Cornall LM, Mathai ML, Hryciw DH, McAinch AJ (2011) Diet-induced obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell Physiol Biochem 28:949–958
Qian MW, Eaton JW (1991) Iron translocation by free fatty acids. Am J Pathol 139:1425–1434
Nassiri-Asl M, Hosseinzadeh H (2009) Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive compounds. Phytother Res 23:1197–1204
Quesada H, del Bas JM, Pajuelo D, Díaz S, Fernandez-Larrea J, Pinent M, Arola L, Salvadó MJ, Bladé C (2009) Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int J Obes (Lond) 33:1007–1012
Padwal RS, Majumdar SR (2007) Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet 369:71–77
Caimari A, del Bas JM, Crescenti A, Arola L (2013) Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int J Obes (Lond) 37:576–583
Stewart LK, Soileau JL, Ribnicky D, Wang ZQ, Raskin I, Poulev A, Majewski M, Cefalu WT, Gettys TW (2008) Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6 J mice fed a high-fat diet. Metabolism 57:S39–S46
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342
Quesada IM, Bustos M, Blay M, Pujadas G, Ardèvol A, Salvadó MJ, Bladé C, Arola L, Fernández-Larrea J (2011) Dietary catechins and procyanidins modulate zinc homeostasis in human HepG2 cells. J Nutr Biochem 22:153–163
Bueno AA, Habitante CA, Oyama LM, Estadella D, Ribeiro EB, Oller do Nascimento CM (2011) White adipose tissue re-growth after partial lipectomy in high fat diet induced obese Wistar rats. J Physiol Sci 61:55–63
Ziemann E, Olek RA, Grzywacz T, Antosiewicz J, Kujach S, Luszczyk M, Smaruj M, Sledziewska E, Laskowski R (2013) Whole-body cryostimulation as an effective method of reducing low-grade inflammation in obese men. J Physiol Sci. doi:10.1007/s12576-013-0269-4
Houschyar KS, Lüdtke R, Dobos GJ, Kalus U, Broecker-Preuss M, Rampp T, Brinkhaus B, Michalsen A (2012) Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: results from a randomized clinical trial. BMC Med 10:54
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Charradi, K., Elkahoui, S., Limam, F. et al. High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: prevention by grape seed and skin extract. J Physiol Sci 63, 445–455 (2013). https://doi.org/10.1007/s12576-013-0283-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-013-0283-6