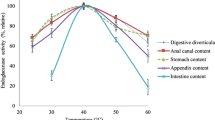
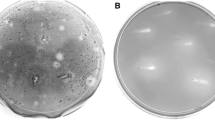
Abstract
Mangrove crabs play a crucial role in the carbon cycle in forests by consuming large amounts of mangrove litter, which is mainly composed of cellulose. However, the detailed mechanism of cellulose digestion remains to be elucidated. We tested endogenous hepatopancreatic cellulase activity in eight species of crabs, including three mangrove sesarmid crabs (Episesarma versicolor, Perisesarma indiarum, and Episesarma palawanense) native to Thailand. Endo-β-1,4-glucanase activity was significantly higher in the enzyme extract from mangrove crabs than in that from Japanese marsh crabs. A β-glucosidase assay revealed particularly high endo-β-1,4-glucanase activity for E. versicolor, whereas little activity was observed for the Japanese marsh crabs. In a zymogram analysis for endo-β-1,4-glucanase activity, endo-β-1,4-glucanase had a similar molecular mass (30.7–33.1 kDa) among the mangrove crabs, whereas various sizes (44.3–84.8 kDa) were found in Japanese crabs depending on the species. These results suggest that mangrove crabs efficiently digest cellulose endogenously.


Similar content being viewed by others
References
Kristensen E (2008) Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J Sea Res 59:30–43
Lee SY (2008) Mangrove macrobenthos: assemblages, services, and linkages. J Sea Res 59:16–29
Cannicci S, Burrows D, Fratini S, Smith TJ III, Offenberg J, Dahdouh-Guebas J (2008) Faunal impact on vegetation structure and ecosystem function in mangrove forests: a review. Aquat Bot 89:186–200
Robertson AI (1986) Leaf–burying crabs: their influence on energy flow and export from mixed mangrove forests (Rhizophora spp.) in north eastern Australia. J Exp Mar Biol Ecol 102:237–248
Micheli F (1993) Feeding ecology of mangrove crabs in North Eastern Australia: mangrove litter consumption by Sesarma messa and Sesarma smithii. J Exp Mar Biol Ecol 171:165–186
Slim FJ, Hemminga MA, Ochieng C, Jannink NT, de la Moriniere EC, van der Velde G (1997) Leaf litter removal by the snail Terebralia palustris (Linnaeus) and sesarmid crabs in an east African mangrove forest (Gazi Bay, Kenya). J Exp Mar Biol Ecol 215:35–48
Lee SY (1997) Potential trophic importance of the faecal material of the mangrove sesarmine crab Sesarma messa. Mar Ecol Prog Ser 159:275–284
Thongtham N, Kristensen E (2005) Carbon and nitrogen balance of leaf-eating sesarmid crabs (Neoepisesarma versicolor) offered different food sources. Estuar Coast Shelf Sci 65:213–222
Giddins RL, Lucas JS, Neilson MJ, Richards GN (1986) Feeding ecology of the mangrove crab Neosarmatium smithi (Crustacea: Decapoda: Sesarmidae). Mar Ecol Prog Ser 33:147–155
Neilson MJ, Richards GN (1989) Chemical-composition of degrading mangrove leaf litter and changes produced after consumption by mangrove crab Neosarmatium smithi (Crustacea, Decapoda, Sesarmidae). J Chem Ecol 15:1267–1283
Watanabe H, Tokuda G (2010) Cellulolytic systems in insects. Annu Rev Entomol 55:609–632
Watanabe H, Noda H, Tokuda G, Lo N (1998) A cellulase gene of termite origin. Nature 394:330–331
Smant G, Stokkermans JP, Yan Y, de Boer JM, Baum TJ, Wang X, Hussey RS, Gommers FJ, Henrissat B, Davis EL, Helder J, Schots A, Bakker J (1998) Endogenous cellulases in animals: isolation of β-1,4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proc Natl Acad Sci USA 95:4906–4911
Sugimura M, Watanabe H, Lo N, Saito H (2003) Purification, characterization, cDNA cloning and nucleotide sequencing of a cellulase from the yellow-spotted longicorn beetle, Psacothea hilaris. Eur J Biochem 270:3455–3460
Lee SJ, Kim SR, Yoon HJ, Kim I, Lee KS, Je YH, Lee SM, Seo SJ, Dae Sohn H, Jin BR (2004) cDNA cloning, expression, and enzymatic activity of a cellulase from the mulberry longicorn beetle, Apriona germari. Comp Biochem Physiol B Biochem Mol Biol 139:107–116
Byrne KA, Lehnert SA, Johnson SE, Moore SS (1999) Isolation of a cDNA encoding a putative cellulase in the red claw crayfish Cherax quadricarinatus. Gene 239:317–324
Nishida Y, Suzuki K, Kumagai Y, Tanaka H, Inoue A, Ojima T (2007) Isolation and primary structure of a cellulase from the Japanese sea urchin Strongylocentrotus nudus. Biochimie 89:1002–1011
Suzuki K, Ojima T, Nishita K (2003) Purification and cDNA cloning of a cellulase from abalone Haliotis discus hannai. Eur J Biochem 270:771–778
Sakamoto K, Touhata K, Yamashita M, Kasai A, Toyohara H (2007) Cellulose digestion by common Japanese freshwater clam Corbicula japonica. Fish Sci 73:675–683
Linton SM, Greenaway P (2004) Presence and properties of cellulase and hemicellulase enzymes of the gecarcinid land crabs Gecarcoidea natalis and Discoplax hirtipes. J Exp Biol 207:4095–4104
Allardyce BJ, Linton SM (2008) Purification and characterisation of endo-β-1,4-glucanase and laminarinase enzymes from the gecarcinid land crab Gecarcoidea natalis and the aquatic crayfish Cherax destructor. J Exp Biol 211:2275–2287
Allardyce BJ, Linton SM, Saborowski R (2010) The last piece in the cellulase puzzle: the characterisation of β-glucosidase from the herbivorous gecarcinid land crab Gecarcoidea natalis. J Exp Biol 213:2950–2957
Boon PY, Daren CJY, Todd PA (2008) Feeding ecology of two species of Perisesarma (Crustacea: Decapoda: Brachyura: Sesarmidae) in Mandai mangroves, Singapore. J Crustacean Biol 28:480–484
Carpenter KE, Niem VH (1998) FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. In: Carpenter KE, Niem VH (eds) Cephalopods, crustaceans, holothurians and sharks, vol 2. FAO, Rome, pp 687–1396
Doi H, Matsumasa M, Toya T, Satoh N, Mizota C, Maki Y, Kikuchi E (2005) Spatial shifts in food sources for macrozoobenthos in an estuarine ecosystem: carbon and nitrogen stable isotope analyses. Estuar Coast Shelf Sci 64:316–322
Okamoto K, Kurihara Y (1989) Feeding habit and food selection of the grapsid crab Hemigrapsus penicillatus. Jpn J Ecol 39:195–202
Kitami T, Honma Y (1981) Notes on the behavior of the land crab, Sesarma haematocheir (De Haan), on the coast of Sado Island in the sea of Japan. Res Crustac 11:113–123
Mchenga ISS, Tsuchiya M (2010) Feeding choice and the fate of organic materials consumed by sesarma crabs Perisesarma bidens (De Haan) when offered different diets. J Mar Biol. Article ID 201932. doi:10.1155/2010/201932
Poon DYN, Chan BKK, Williams GA (2010) Spatial and temporal variation in diets of the crabs Metopograpsus frontalis (Grapsidae) and Perisesarma bidens (Sesarmidae): implications for mangrove food webs. Hydrobiologia 638:29–40
Mfilinge PL, Tsuchiya M (2008) Effect of temperature on leaf litter consumption by grapsid crabs in a subtropical mangrove (Okinawa, Japan). J Sea Res 59:94–102
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Jue C, Lipke P (1985) Determination of reducing sugars in the nanomole range with tetrazolium blue. J Biochem Biophys Methods 11:109–115
Xue XM, Anderson AJ, Richardson NA, Anderson AJ, Xue GP, Mather PB (1999) Characterisation of cellulase activity in the digestive system of the redclaw crayfish (Cherax quadricarinatus). Aquaculture 180:373–386
Crawford AC, Kricker JA, Anderson AJ, Richardson NR, Mather PB (2004) Structure and function of a cellulase gene in redclaw crayfish, Cherax quadricarinatus. Gene 340:267–274
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adachi, K., Toriyama, K., Azekura, T. et al. Potent cellulase activity in the hepatopancreas of mangrove crabs. Fish Sci 78, 1309–1314 (2012). https://doi.org/10.1007/s12562-012-0547-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-012-0547-8