Abstract
Background
Multi-component vocational rehabilitation (VR) provides positive short-term outcomes in patients with prolonged fatigue.
Purpose
The purpose of this study is to evaluate the long-term outcomes of Dutch multi-component VR up to 18 months after treatment.
Method
In a pre–post-study, measurements were taken before treatment (t0), after treatment (t1) and in long-term follow-ups at 6 (t2), 12 (t3) and 18 months (t4) after treatment. Primary outcomes (fatigue, work participation and workability) and secondary outcomes [physical and social functioning, mental health and heart rate variability (HRV)] were assessed over time using linear mixed models analyses. Post hoc long-term outcomes were compared with t0 and t1.
Results
Sixty patients with severe fatigue complaints participated. The primary outcomes significantly (p < 0.001) improved at follow-ups compared with t0 and showed no relapse compared with t1. Moreover, fatigue decreased (p < 0.002) whereas workability (p < 0.001) and work participation (p < 0.001) increased further after treatment (t1). The secondary outcomes, physical functioning, mental health, social functioning and HRV, improved significantly (p < 0.001, p < 0.001, p < 0.001 and p = 0.049, respectively) over the long term compared with t0. At 6-month follow-up (t2), mental health (p < 0.003) and social functioning (p = 0.003) further increased after the treatment was stopped.
Conclusion
Multi-component VR treatments seem to significantly and in a clinically relevant way decrease fatigue symptoms and improve individual functioning and work participation in patients with severe prolonged fatigue over the long term and without showing relapse.
Similar content being viewed by others
Introduction
Prolonged fatigue is highly prevalent in the general population [1, 2] and is also a prominent symptom in chronic diseases such as rheumatoid arthritis [3], cancer [4] and depressive disorders [5]. In addition, fatigue can be a discrete disorder (i.e. chronic fatigue syndrome (CFS)) [6] or occur independent of a specific chronic disease or disorder (i.e. common health problem) [7]. In all cases, in addition to individual suffering, prolonged fatigue can affect social and occupational functioning that may result in negative consequences concerning work capacity. Sickness absence due to prolonged fatigue may result in poor quality of life [8] and serious economic consequences [9]. If effective treatments for workers who are disabled from prolonged fatigue were available, work participation and health could be improved.
Because of its multifactorial origin, the biopsychosocial model [10], which states that sickness and health result from a complex interaction between biological, psychological and social factors, seems appropriate to explain prolonged fatigue [11, 12]. In line with this model, mechanisms responsible for the perpetual nature of prolonged fatigue have been investigated. From a biological perspective, prolonged fatigue is related to dysregulation of physiological stress systems due to overuse of these systems [11, 13, 14]. In addition, cognitive and behavioural factors (e.g. causal attribution, low self-efficacy, dysfunctional beliefs about activity and fatigue) are also involved in the perpetuation of fatigue complaints [15–17]. Furthermore, lack of social support can be a perpetuating factor in fatigued patients [18]. To stimulate effective recovery in fatigued patients, treatments should take individual perpetuating factors into account [19, 20]. In the Netherlands, outpatient vocational rehabilitation (VR) institutions guide workers with prolonged fatigue and functional limitations. These institutions use multi-component treatments to increase physical and mental functioning, and facilitate work participation. These real-life practices have been evaluated before, using process and outcome measures in a population of disabled fatigued patients recruited from the practice setting [21]. This kind of research can be identified as practice-based research and is suitable for evaluating multi-component treatments in real-life settings in an everyday patient population [22, 23]. In addition, these multi-component treatments showed significant and clinically relevant outcomes in symptomatic and functional improvements and work participation up to 3 months after treatment [21]. However, it is unclear whether these positive short-term outcomes will be sustained over time. Long-term outcomes of treatments are of special interest because of the long-lasting character of fatigue complaints; the perpetuating factors often lead to long-lasting and deeply ingrained patterns [12]. Moreover, multi-component interventions that target behavioural change, by intervening with perpetuating factors such as dysfunctional beliefs and coping strategies in work and private life, may have the potential to be effective in the long term (1 year after discharge of treatment) [24]. Therefore, we evaluated the long-term outcomes of VR treatments in the Netherlands in patients with prolonged fatigue complaints and participation problems. The purpose of this study is to answer the following question: What are the outcomes up to 18 months after VR treatment on fatigue symptoms, work participation, work ability, daily functioning and in physiological parameters (heart rate variability) in patients with prolonged fatigue?
Method
Patients
Our target population consisted of patients who enrolled in three participating outpatient institutions for VR treatments from 2006 to 2008. The outpatient institutions used specific inclusion criteria to select clients before treatment, including the following: good command of the spoken and written Dutch, motivation to take part in the treatment, complaints for (in general) more than 3 months and no diagnosis of a psychiatric disorder. The inclusion criteria to participate in this study were as follows: aged between 18 and 60 years, fatigue complaints as a main or important symptom and suffering from functional impairments (i.e. constraints in everyday life) due to fatigue complaints. Eligible patients were approached and informed about the study before they provided written consent. This study was approved by the medical ethical committee of the Academic Medical Center.
Design
In this practice-based research, we evaluated VR treatments in their natural setting [22, 23] and used the TREND (Transparent Reporting of Evaluations with Non-randomised Designs) statement [25] in designing and reporting this study. We used a pre–post-design with repeated measurements at baseline (t0), upon completion of the treatment (t1) and 3 (t′), 6 (t2), 12 (t3) and 18 (t4) months follow-up. The study participants were recruited in two phases. First, patients were asked to participate in the study until 6 months after completing the treatment. After 6 months, we asked if patients would further participate and complete questionnaires at 12 and 18 months after completion. In the current study, we focused on the long-term outcomes (6, 12 and 18 months after completion) and report outcomes at these follow-up measurement compared to t0 and t1. The short-term outcomes of the VR treatments (t1 and t′ versus t0) are described in a previous publication [21].
VR Treatments
The VR treatments were provided by three existing outpatient institutions in the Netherlands. These institutions are focused on patients with fatigue complaints who were on sick leave and/or had limitations in work function. Usually patients were referred by occupational physicians or self-referred (e.g. advised to visit the institution by people in their social environment and/or other caregivers). The main aim of the VR treatments was to improve individual and occupational functioning in patients with prolonged fatigue complaints by achieving a normal balance between activity and rest, and subsequently between daily life and work. From previous research, we know that these three institutions use a biopsychosocial-based multi-component treatment [21]. That is, these treatments consisted of biological/physical components, psychological/cognitive behavioural components and social/work-directed components.
Biological/Physical Component
Physical training included an individualised progressive personal workout scheme based on daily heart rate levels or a graded exercise programme using time-contingent training. Physical training was guided by a movement specialist and/or physiotherapist and exercises were done on a bicycle, treadmill, cross trainer and/or power station. Physical training was aimed at improving physical fitness, increasing activity levels and body awareness. Relaxation and breathing exercises were provided in an attempt to reduce stress and increase body awareness.
Psychological Component
Group and individual sessions with a psychologist or personal/mental coach used cognitive–behavioural principles aimed at relieving distress, increasing illness knowledge and raising awareness of perceptions, attitudes, and beliefs. Improving coping strategies and changing dysfunctional behaviour were goals as well.
Social/Work-Directed Component
Return-to-work (RTW) sessions (individual or group sessions) with a psychologist or occupational expert addressed patients’ attitudes towards work, job conditions and work adaptations, and social environment (partner). In addition, a patient-tailored phased RTW plan was made, in which RTW (e.g. number of working hours, work task and work demands) was gradually increased. These sessions were intended to increase awareness of behavioural patterns at work and in private life, improve coping skills and facilitate work participation. The VR treatments took in total 4 to 18 weeks with a visit frequency of three to five times per week in the first part of the treatment period (1/3) and decreased from two times in the second part to one time per week in the third part of the treatment period. For detailed information about the content of the VR treatments, see Joosen et al. [21].
The specific content of the VR treatment (e.g. number of sessions, topics focussed upon, duration of the treatment) can differ somewhat between patients in this study because the treatment is provided by three different institutions. Indeed, the three institutions pursue the same goal in a population of disabled fatigued patients using the same approach but with a focus on the content that is tailored to meet individual needs [21]. According to practice-based research methodology [22, 23, 26], we aimed at evaluating real-world practice. Therefore we decided to combine the three treatments and report on the long-term outcomes of the total group of patients.
Primary Outcome Measures
Primary outcome measures were degree of fatigue, work participation, and work ability. To assess fatigue, three questionnaires were used. First, fatigue complaints were measured using the Checklist Individual Strength (CIS) [27], which consists of 20 statements. The CIS has been validated in the Dutch working population [28]. Second, we used the vitality subscale of the Dutch version of the RAND-36 Health survey [29], which is almost identical to the MOS SF-36 [30] and is a reliable and validated generic instrument [29]. Third, work-related fatigue was measured with the Need for Recovery After Work scale [31], with 11 yes/no items. The Need for Recovery scale was found reliable and valid in a working population [32, 33].
Data on work participation were collected by researcher-formulated questions at different measurement times. Data consisted of the following: (1) current work status, in terms of employed or unemployed; (2) number of contractual working hours; and (3) absolute number of hours the patient was working at that moment. With these data we determined the following: (1) percentage of return to original work, defined as the mean percentage of return working to the original contracted working hours at t0, and (2) percentage of return to work, defined as the mean percentage of return working to the contracted working hours at the measurement time [34]. In this latter variable, changes in contracted working hours during the study period were taken in account.
Self-reported work ability was assessed using two items from the Work Ability Index [35]: (1) currently perceived work ability compared with lifetime best, scoring between 0 (‘not being able to work’) and 10 (‘lifetime best work ability’), and (2) personal prognosis of work ability in the next 2 years, scored on a three-point scale (‘hardly able to work’, ‘not sure’ and ‘fairly sure to be able to work’).
Secondary Outcome Measures
The secondary outcome measures included physical functioning, mental health, social functioning and heart rate variability (HRV). Subscales of the RAND-36 Health survey [29] were used to measure physical functioning and physical role limitation, mental health and emotional role limitation, and social functioning.
HRV was used as a physiological indicator that reflects sympathetic and parasympathetic activity of the autonomic nervous system. Prolonged exposure to stress can lead to dysregulation of this system (i.e. lower parasympathetic activity) [14, 36] and can be identified by decreased HRV [37, 38]. HRV was recorded using the Co2ntrol (Decon Medical Systems, Weesp, the Netherlands), a small device attached to a chest strap that detects beat-to-beat intervals [39]. Due to practical constraints, HRV was only measured at t0, t1 and t2 during a standardised test protocol: 5 min seated in a resting position for adaptation, followed by 12 min light exercise on a bicycle ergometer using a single load of 50 W with a pedal frequency between 60 and 65 min−1. The Co2ntrol was developed according to the guidelines of the European and North American Task Force [40]. It was found to provide reproducible HRV measurements in healthy individuals [41] and in patients with prolonged fatigue [42].
In addition, personal demographics, duration of fatigue symptoms and duration of functional impairments were obtained by questionnaires before treatment (patient characteristics at baseline). Furthermore, additional treatments and sick leave duration were recorded at two points in time (t3 and t4). At follow-up, patients were asked to report which health care providers they had visited concerning fatigue complaints during the last 6 months to monitor additional treatments. Next, sick leave duration during the last 6 months was recorded.
Data Reduction
Data from the questionnaires were reduced and/or transformed into total scores. In CIS data, we added the item scores to a total score ranging from 20 to 140. Higher scores indicated a higher severity of fatigue. Item scores from the Need for Recovery scale were added and transformed [29] into a scale ranging from 0 to 100. Higher scores indicated a higher degree of need for recovery after work. RAND-36 scores were added separate per scale and transformed [29] into a score ranging from 0 to 100; higher scores represent better outcomes.
To define HRV, raw data were transferred to HRV Analysis Software version 2.0 (http://venda.uku.fi/research/biosignal) and data artefacts were detected and processed by the software. The data were de-trended using the smoothn prior option. To determine the spectrum of HRV, the fast Fourier transform option was used and data were re-sampled at a rate of 4 Hz using cubic interpolation. The final 9 min of the 12-min recording period during light exercise were selected. HRV was measured by means of heart period high-frequency (HF) power. HF power values were used to estimate respiratory sinus arrhythmia (RSA), the variability of heart period in the respiratory frequency band. RSA is considered a valid index of changes in cardiac vagal tone, which interacts with parasympathetic activity [38, 43]. In this study, HF power was computed in the 0.15–0.5-Hz respiration window. The VR treatments were expected to have beneficial effects on physiological status (i.e. raise HF power after completion of the interventions).
Statistical Analysis
Our target sample size was 110 patients from the first phase of the evaluation study [21]. Because we knew that part of this sample dropped out of the study after the first phase, we performed a non-response analysis to check for possible bias. Differences in baseline characteristics and primary short-term outcomes (i.e. degree of fatigue and return to work) were tested with t tests for continuous variables and chi-tests for categorical variables.
To analyse the long-term outcomes of the variables over time up to 18 months, we performed linear mixed model analyses based on repeated measures. The best fitting covariance–variance model was tested before the analysis was applied. If a statistically significant overall effect was found, post hoc tests using Bonferroni correction (taking into account the multiple comparisons) were performed to compare outcomes between follow-up measurements and t0 and t1. To assess the changes in HRV between t0 and t1 and between t0 and t2, non-parametric Wilcoxon signed rank tests were carried out. Statistical analyses were performed using SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Values of p < 0.05 were considered statistically significant. Post hoc values in primary and secondary outcome measures were considered significant at p < 0.0083 due to Bonferroni corrections (p value divided by the number of comparisons, i.e. six).
Results
Patient Characteristics
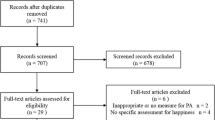
A total of 110 patients, 50 men and 60 women, enrolled in this study and 60 patients completed the 18-month follow-up measurement (Fig. 1). Reasons for failure to follow-up are presented in Fig. 2. Only ‘completers’, 60 patients (28 men and 32 women) who completed the 18-month follow-up measurement, were included in the analysis. Patients had, on average, severe disabling fatigue complaints for many years (mean 3.4 years). Most patients (73%) were partly or fully on sick leave, 17% were working full time and 10% had no paid job (Table 1).
Primary outcomes: fatigue severity, work-related fatigue, vitality return to original work, return to work and work ability. Mean scores (95% confidence intervals) on primary outcomes at t0 (baseline), t1 (at completion of the treatment), t2 (6 months follow-up), t3 (12 months follow-up) and t4 (18 months follow-up) and p values for the differences between follow-up measures and t1. *p < 0.001, significant post hoc outcome within subjects compared with t0. Significant when p < 0.0083 (Bonferroni corrected). ^p < 0.0083, significant post hoc outcome within subjects compared with t1 (p < 0.0083 Bonferroni corrected)
We performed a non-response analysis and compared ‘completers’ with ‘non-completers’. No significant between group difference was found on gender, age, duration of fatigue complaints, duration of functional impairments and fatigue severity at baseline. ‘Completers’ and ‘non-completers’ did not differ in fatigue severity levels (i.e. mean CIS scores) between t1 and t0, nor did the groups differ in return-to-original work percentage between t1 and t0.
Half of the 60 patients (52%) volunteered to participate in various additional treatments between 6 and 18 months after completing the VR treatments. Many of these patients (21–23%) were treated by general practitioners and medical specialists or took extra sessions in the VR institution. However, most patients (61%) sought care in the complementary and alternative medicine field (including acupuncture, homeopathy and orthomolecular medicine). Type and number of additional treatments are reported in Table 2.
In the period between 6 and 18 months after treatment, 17 patients (28%) had not been on sick leave for 1 year. In this period, 14 patients (24%) had been on sick leave due to fatigue complaints. The mean duration of sick leave was 9 weeks, ranging from 2 days to 1 year. Two patients were on sick leave for the full 1-year period (data not shown).
Primary Outcome Measures
Figure 2 shows the results (mean scores, confidence intervals and p values) of primary outcomes. Fatigue severity, work-related fatigue, vitality, percentage of return to original work, percentage of return to work and perceived work ability all improved significantly (p < 0.001) over time. In post hoc analyses, all outcomes improved significantly (p < 0.001) in follow-up measures compared to baseline. In addition, the percentage of cases with chronic fatigue (CIS score above 76) [44] decreased from 87% at baseline to 46% after treatment and to 37% at 18 months follow-up.
Compared to t1, long-term improvements were less clear. No relapse in the assessed outcomes was found at 6, 12 and 18 months after treatment compared to t1. Moreover, two out of three fatigue outcomes significantly improved at 6 months after treatment (t2) compared to t1 [work-related fatigue (p = 0.002) and vitality (p = 0.004)], as did work ability (p < 0.001). In addition, both work participation long-term improvements (t2, t3 and t4) were significant (p < 0.001) compared to t1.
Out of 60 patients, 28 (47%) stated at baseline that they were ‘fairly sure’ they would be able to work in 2 years. At t1, this number rose to 44 patients (73%). At 12 and 18 months follow-up, 39 out of 53 patients (74%) and 44 out of 58 patients (76%), respectively, were ‘fairly sure’ they would be able to work in 2 years (data not shown).
Secondary Outcome Measures
Table 3 reports mean scores, confidence intervals and overall p values in the secondary outcomes per measurement. Five secondary outcomes (physical functioning, physical role limitation, mental health, emotional role limitation and social functioning) improved significantly (p < 0.001) over time. These outcomes improved significantly (p < 0.001) at all follow-up measures compared to baseline.
In post hoc analyses compared to t1, non-significant long-term improvements at t2, t3 and t4 were found in physical functioning (p = 0.470, p = 0.239 and p = 0.396, respectively) and physical role functioning (p = 0.012, p = 0.035 and p = 0.041, respectively). In mental health, mean scores at 6 months after treatment improved significantly (p < 0.001) and emotional role limitation improved significantly 6 (p = 0.003) and 12 months (p = 0.004) after treatment compared to t1. Social functioning increased significantly as well after the treatment was stopped (p = 0.003 at 6 months follow-up compared to t1).
For heart rate variability, compared with baseline, HF power values improved significantly (p < 0.001) after treatment and at 6 months follow-up (p = 0.049). However, HF power did not improve significantly at 6-month follow-up compared with t1 (p = 0.119, Table 4).
Discussion
The results of our study suggest that in patients with prolonged fatigue, multi-component VR treatments significantly decrease fatigue symptoms and improve work participation, work ability, and physical, mental, and social functioning over the long term. In addition, HRV as a physiological parameter did improve 6 months after the VR treatments. The results show that the positive changes in patients measured immediately after completing the treatment were maintained over the long term. Moreover, positive changes for fatigue complaints, work participation, work ability, mental health and social functioning improved further up to 18 months after treatment.
Not surprisingly, the largest improvements compared to baseline were found immediately after completing the VR treatment. More surprisingly, the results show that short-term improvements are maintained over the long term, after treatment was stopped. These long-term improvements can be interpreted as clinically relevant outcomes, that is Cohen’s effect size statistic d > 0.50 [45], due to large effect sizes (d > 1.2) in all outcome measurements. First, the three fatigue measures show the same pattern of recovery. Moreover, mean fatigue severity scores (CIS) and mean scores in work-related fatigue decreased below the cut-off points of these scales, indicating chronic fatigue (mean = 76) [44] and high risk of receiving treatment (mean = 55), respectively [46, 47]. Despite these significant and clinically relevant results, after 18 months patients in this study reported still more fatigue complaints than the Dutch (working) population (mean CIS score = 47 and vitality score = 69) [44, 48]. Second, both work participation measures improved further after treatment. Eighteen months after completing the treatment, return to original work rose to 87%. That means that on average, patients worked for 87% of their contract hours at baseline. This result is similar to studies reporting RTW after multi-component treatment in upper extremity complaints [34] and in previous retrospective data [49]. Taking into account that some patients (n = 15) modified their employment contract (i.e. less contracted hours) during the study period, return to work rose to 98% at 18 months follow-up. The VR treatment was aimed at facilitating full RTW by using work-directed components. However, another important aim of the treatment was to increase awareness of attitudes towards work and improve coping strategies and behaviour. In addition, patients have indicated that their work participation increases by learning to recognise their behavioural patterns at work and in private lives, and training to cope with their limitations and capacities (e.g. setting boundaries) [50]. As a result of gaining this insight, patients may have changed their employment contract to avoid exceeding their capacities, thus resulting in a higher return-to-work percentage. It should be noted that eight patients were not included in the return-to-work analysis because they had no paid job anymore at 18 months follow-up. Because we do not know why these patients lost their employment contract (e.g. retirement, going back to college, permanent work disability) and if this job loss was the result of attending the VR treatment, we cannot draw conclusions from this.
The secondary outcomes, physical functioning, mental health and social functioning, showed long-term improvements. Physical functioning mean scores at 18 months follow-up were higher than scores in the Dutch population (mean = 83) [48]. Mental health and social functioning improved significantly after the treatment was stopped. Eighteen months after treatment, mental health scores moved towards the scores found in the Dutch population (mean = 77) [48], but social functioning scores stayed below the scores of the Dutch population (mean = 84). As for HRV, we found 6-month follow-up (t2) improvements compared with baseline. Mean HF power worsened compared with t1, however not significantly. Few, if any, studies have investigated the effects of vocational interventions on HRV in fatigued patients. Though, we do know that physical exercise that is focussed on physiological adaptation improves HF power in other populations (e.g. healthy and coronary patients) over the short term [51, 52]. However, these effects may be maintained only if the exercise is continued. We do not know if the patients in our study continued physical training in their private time.
The main aim of the VR treatments was to improve individual and occupational functioning by achieving a normal balance between activity and rest, and subsequently between daily life and work. To achieve this normal balance, perpetuating factors that hindered recovery were addressed from a biopsychosocial perspective. The complexity of the treatment and outcomes, that is a combination of body awareness, increased physical fitness, awareness of attitudes and beliefs towards work and in private life, improving coping strategies and making a gradual RTW plan, is thought to increase daily functioning and, moreover, change patients’ behaviour (e.g. adopt a new lifestyle). Consequently, this would improve participation over the long term. The current results show that although fatigue symptoms remain prevalent, outcomes on functioning in daily life and work participation improve over the long term and improve further after treatment is stopped. This suggests that patients are better capable of coping with perceived complaints in their private and working lives. These findings are supported by the findings of patients’ RTW experiences after treatment in this same population (Joosen et al., submitted). In addition, in upper extremity musculoskeletal disorders [34] and in low back pain [53], biopsychosocial rehabilitation showed positive effects on work participation over the long term (1 year follow-up).
This study was designed as a practice-based research attempting to answer a question that originates from everyday practice. In the Netherlands, outpatient VR care is decontrolled. Thus, various treatments are designed by outpatient institutions and carried out by experienced trainers, caregivers and supervisors. It is of great importance to evaluate the outcomes of these everyday practices, as many fatigued patients who seek help attend these treatments. However, conducting a randomised controlled study in this setting was not feasible; among other reasons, institutions were bound by contracts with employers. In practice-based research, the content of the intervention and patients participating in the study should be the same as in “clinical” practice. Next, a broad range of real-life outcomes should be collected. These factors were taken into account, which gives this study high external validity [22, 26, 54]. Due to the absence of a control group, which is not unusual in practice-based research, a number of measures were taken to strengthen the design (and thereby internal validity) according to the TREND statement [25]. The content of VR treatment was theoretically specified, outcomes were selected based on the interventions, and measured using reliable and validated outcome variables. The results of this study are in line with and confirmed by different perspectives by longitudinal data (current study), process evaluation (described in [21]) and patients’ perspectives [50]. In addition, considering the long-standing nature of fatigue complaints and perceived disabilities, spontaneous recovery of these problems was unlikely. Considering these points, we believe that the outcomes of this study may be attributed to the VR treatment provided by the outpatient institutions in a population of prolonged fatigue patients with participation problems. It would, however, be interesting to repeat this study in a population of workers specifically diagnosed with CFS.
We conclude that multi-component VR treatments have the potential to reduce fatigue symptoms and improve individual functioning and work participation in patients with severe prolonged fatigue complaints over the long term without relapses. It is therefore recommended to use multi-component VR treatments with a biopsychosocial approach for patients with disabling prolonged fatigue complaints. These results are of importance to the occupational health field in preventing and managing sickness absence in fatigued workers through referral to VR treatments.
References
Bültmann U, Kant I, Kasl SV, Beurskens AJ, van den Brandt PA. Fatigue and psychological distress in the working population: psychometrics, prevalence, and correlates. J Psychosom Res. 2002;52:445–52.
Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJ, Wessely SC. Population based study of fatigue and psychological distress. BMJ. 1994;308:763–6.
Lacaille D, White MA, Backman CL, Gignac MA. Problems faced at work due to inflammatory arthritis: new insights gained from understanding patients' perspective. Arthritis Rheum. 2007;57:1269–79.
Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev. 2009;CD006953.
Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8:93–105.
Lewis G, Wessely S. The epidemiology of fatigue: more questions than answers. J Epidemiol Community Health. 1992;46:92–7.
Barnes MC, Buck R, Williams G, Webb K, Aylward M. Beliefs about common health problems and work: a qualitative study. Soc Sci Med. 2008;67:657–65.
van de Mheen H, Stronks K, Schrijvers CT, Mackenbach JP. The influence of adult ill health on occupational class mobility and mobility out of and into employment in the The Netherlands. Soc Sci Med. 1999;49:509–18.
Henriksson CM, Liedberg GM, Gerdle B. Women with fibromyalgia: work and rehabilitation. Disabil Rehabil. 2005;27:685–94.
Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–36.
Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst Rev 2008;CD001027.
Van Houdenhove B, Luyten P. Customizing treatment of chronic fatigue syndrome and fibromyalgia: the role of perpetuating factors. Psychosomatics. 2008;49:470–7.
Cleare AJ. The neuroendocrinology of chronic fatigue syndrome. Endocr Rev. 2003;24:236–52.
McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9.
Huibers MJ, Bleijenberg G, van Amelsvoort LG, Beurskens AJ, van Schayck CP, Bazelmans E, et al. Predictors of outcome in fatigued employees on sick leave: results from a randomised trial. J Psychosom Res. 2004;57:443–9.
Vercoulen JH, Swanink CM, Galama JM, Fennis JF, Jongen PJ, Hommes OR, et al. The persistence of fatigue in chronic fatigue syndrome and multiple sclerosis: development of a model. J Psychosom Res. 1998;45:507–17.
Knoop H, Prins JB, Moss-Morris R, Bleijenberg G. The central role of cognitive processes in the perpetuation of chronic fatigue syndrome. J Psychosom Res. 2010;68:489–94.
Prins JB, Bos E, Huibers MJ, Servaes P, van der Werf SP, van der Meer JW, et al. Social support and the persistence of complaints in chronic fatigue syndrome. Psychother Psychosom. 2004;73:174–82.
Van Houdenhove B, Luyten P. Treatment of chronic fatigue syndrome: how to find a ‘new equilibrium’? Patient Educ Couns. 2009;77:153–4.
Deary V, Chalder T, Sharpe M. The cognitive behavioural model of medically unexplained symptoms: a theoretical and empirical review. Clin Psychol Rev. 2007;27:781–97.
Joosen M, Frings-Dresen M, Sluiter J. Process and outcome evaluation of vocational rehabilitation interventions in patients with prolonged fatigue complaints. Int J Behav Med. 2011;18:160–71.
Hotopf M. The pragmatic randomised controlled trial. Adv Psychiatr Treat. 2002;8:326–33.
Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–32.
Landsman-Dijkstra JJ, van Wijck R, Groothoff JW. The long-term lasting effectiveness on self-efficacy, attribution style, expression of emotions and quality of life of a body awareness program for chronic a-specific psychosomatic symptoms. Patient Educ Couns. 2006;60:66–79.
Des Jarlais DC, Lyles C, Crepaz N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94:361–6.
Horn SD, Gassaway J. Practice based evidence: incorporating clinical heterogeneity and patient-reported outcomes for comparative effectiveness research. Med Care. 2010;48:S17–22.
Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38:383–92.
Beurskens AJ, Bültmann U, Kant I, Vercoulen JH, Bleijenberg G, Swaen GM. Fatigue among working people: validity of a questionnaire measure. Occup Environ Med. 2000;57:353–7.
van der Zee KI, Sanderman R. Het meten van de algemene gezondheidstoestand met de RAND-36. Een handleiding. (Measuring general health status with the RAND-36. Users Manual). Rijksuniversiteit Groningen: Noordelijk Centrum voor Gezondheidsvraagstukken 1993.
Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83.
van Veldhoven M, Broersen S. Measurement quality and validity of the “need for recovery scale”. Occup Environ Med. 2003;60 Suppl 1:i3–9.
de Croon EM, Sluiter JK, Frings-Dresen MH. Psychometric properties of the Need for Recovery after work scale: test–retest reliability and sensitivity to detect change. Occup Environ Med. 2006;63:202–6.
Sluiter JK, de Croon EM, Meijman TF, Frings-Dresen MH. Need for recovery from work related fatigue and its role in the development and prediction of subjective health complaints. Occup Environ Med. 2003;60 Suppl 1:i62–70.
Meijer EM, Sluiter JK, Heyma A, Sadiraj K, Frings-Dresen MH. Cost-effectiveness of multidisciplinary treatment in sick-listed patients with upper extremity musculoskeletal disorders: a randomized, controlled trial with one-year follow-up. Int Arch Occup Environ Health. 2006;79:654–64.
Tuomi K, Ilmarinen J, Jahkola A, Katajarinne L, Tulkki A. Work Ability Index. Helsinki: Finnish Institute of Occupational Health 1997.
Langelaan S. Burnout and work engagement: exploring individual and psychophysiological differences. The Netherlands: University of Utrecht; 2009.
Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–41.
Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–48.
Joosen M, Sluiter J, Joling C, Frings-Dresen M. Evaluation of the effects of a training programme for patients with prolonged fatigue on physiological parameters and fatigue complaints. Int J Occup Med Environ Health. 2008;21:237–46.
The Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–81.
Guijt AM, Sluiter JK, Frings-Dresen MH. Test–retest reliability of heart rate variability and respiration rate at rest and during light physical activity in normal subjects. Arch Med Res. 2007;38:113–20.
Sluiter JK, Guijt AM, Frings-Dresen MH. Reproducibility and validity of heart rate variability and respiration rate measurements in participants with prolonged fatigue complaints. Int Arch Occup Environ Health. 2009;82:623–30.
Langelaan S, Bakker AB, Schaufeli WB, van Rhenen W, van Doornen LJ. Is burnout related to allostatic load? Int J Behav Med. 2007;14:213–21.
Bültmann U, de Vries M, Beurskens AJ, Bleijenberg G, Vercoulen JH, Kant I. Measurement of prolonged fatigue in the working population: determination of a cutoff point for the checklist individual strength. J Occup Health Psychol. 2000;5:411–6.
Cohen J. Statistical power analyses for the behavioural sciences. New York: Academic Press; 1977.
van Veldhoven MJ, Sluiter JK. Work-related recovery opportunities: testing scale properties and validity in relation to health. Int Arch Occup Environ Health. 2009;82(9):1065–75.
Verdonk P, Hooftman WE, van Veldhoven MJ, Boelens LR, Koppes LL. Work-related fatigue: the specific case of highly educated women in the Netherlands. Int Arch Occup Environ Health. 2010;83:309–21.
Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–68.
Joosen MC, Stal W, Swijenburg RH, Sluiter JK, Frings-Dresen MH. Evaluatie van een multidisciplinair vitaliteitsprogramma bij aanhoudende vermoeidheidsklachten (Evaluation of the effect of a multidisciplinairy treatment programme on prolonged fatigue). Tijdschrift voor Bedrijfs-en Verzekeringsgeneeskunde. 2007;15:437–43.
Joosen MC, Frings-Dresen MH, Sluiter JK. Work-related limitations and return-to-work experiences in prolonged fatigue: workers' perspectives before and after vocational treatment. Disabil Rehabil. 2011;33(23–24):2166–78.
Sandercock GR, Bromley PD, Brodie DA. Effects of exercise on heart rate variability: inferences from meta-analysis. Med Sci Sports Exerc. 2005;37:433–9.
Sandercock GR, Grocott-Mason R, Brodie DA. Changes in short-term measures of heart rate variability after eight weeks of cardiac rehabilitation. Clin Auton Res. 2007;17:39–45.
Karjalainen K, Malmivaara A, van Tulder M, Roine R, Jauhiainen M, Hurri H, Koes B. Multidisciplinary biopsychosocial rehabilitation for subacute low back pain among working age adults. Cochrane Database Syst Rev 2003;CD002193.
Kersten P, Ellis-Hill C, McPherson KM, Harrington R. Beyond the RCT—understanding the relationship between interventions, individuals and outcome—the example of neurological rehabilitation. Disabil Rehabil. 2010;32:1028–34.
Acknowledgements
This study was financially supported by the Stichting Instituut Gak. The authors would like to thank all patients who participated in this study and are grateful for the involvement of the outpatient institutions providing the treatments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Joosen, M.C.W., Frings-Dresen, M.H.W. & Sluiter, J.K. Long-Term Outcomes Following Vocational Rehabilitation Treatments in Patients with Prolonged Fatigue. Int.J. Behav. Med. 20, 42–51 (2013). https://doi.org/10.1007/s12529-011-9208-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12529-011-9208-z