Abstract
Introduction
Intradermal (ID) influenza vaccination induces an enhanced immune response in the elderly when compared with intramuscular (IM) vaccination. In 2009, an ID seasonal influenza vaccine (Intanza® [Sanofi Pasteur MSD, Lyon, France] 15 μg) was approved for use in individuals aged ≥ 60 years in Europe. This survey conducted in Belgium was the first in Europe to assess the acceptability of this vaccine in routine clinical practice by vaccinees and their general practitioners (GPs).
Methods
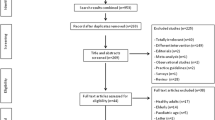
GPs willing to use both the ID and IM influenza vaccines were selected during the 2010–2011 influenza season. Each GP recruited ≤ 10 patients aged ≥ 60 years who received the ID vaccine. Vaccinees and GPs completed questionnaires about their opinions on influenza vaccination and the acceptability of the ID influenza vaccine.
Results
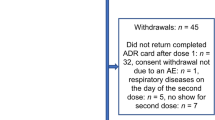
In total, 105 GPs and 837 vaccinees completed questionnaires. A high proportion of vaccinees (40.3%) was aged ≥ 75 years, and 95.5% had been vaccinated the previous year. The majority of vaccinees was very satisfied (70.0%) or satisfied (27.9%) with the ID vaccine. The main reasons for the high satisfaction rate were that the injection was not very painful, administration was quick, and the vaccinee felt confident about the micro-needle injection system. Most vaccinees (91.1%) who had previously received IM influenza vaccination preferred the ID vaccine, and 98.5% of vaccinees reported they would consider receiving the ID vaccine the following year. The majority of GPs was very satisfied (78.6%) or satisfied (18.4%) with the ID vaccine, and most GPs (87.6%) expressed a preference for the ID vaccine over IM influenza vaccine.
Conclusion
The ID influenza vaccine was well accepted by vaccinees and their GPs, who expressed a preference for the ID vaccine over conventional IM influenza vaccine. The availability of the ID influenza vaccine may help to improve uptake of seasonal influenza vaccination in the elderly.
Similar content being viewed by others
References
World Health Organization. Influenza vaccines. Wkly Epidemiol Rec. 2005;80:279–287.
World Health Organization. Fifty-sixth World Health Assembly (WHA). Prevention and control of influenza pandemics and annual epidemics. WHA 10th plenary meeting, 28 May 2003. Available at: apps.who.int/gb/archive/pdf_files/WHA56/ea56r19.pdf. Accessed Jan 5 2012.
Council of the European Union. Council recommendation of 22 December 2009 on seasonal influenza vaccination (2009/1019/EU). Off J Eur Union. 2009;348:71–72. Available at: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:348:0071:0072:EN:PDF. Accessed Jan 5 2012.
Superior Health Council. Publication of the Superior Health Council No. 8682, September 1, 2010. Vaccination against seasonal influenza. Winter Season 2010–2011. [Article in French]. Available at: http://www.health.belgium.be/internet2Prd/groups/public/@public/@shc/documents/ie2divers/19064111_fr.pdf. Accessed May 21 2012.
Lui KJ, Kendal AP. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987;77:712–716.
Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186.
Rizzo C, Bella A, Viboud C, et al. Trends for influenza-related deaths during pandemic and epidemic seasons, Italy, 1969–2001. Emerg Infect Dis. 2007;13:694–69.
Nichol KL, Nordin J, Mullooly J, et al. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332.
Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–1381.
Mereckiene J, Cotter S, D’Ancona F, et al. Differences in national influenza vaccination policies across the European Union, Norway and Iceland 2008–2009. Euro Surveill. 2010;15.pii:19700.
De Bruyn K, Saevels J. Influenza vaccination in community pharmacies 2009/2010. J Pharm Belg. 2010;3:75–80. (Article in French).
De Bruyn K, Hamelinck W. Influenza vaccines dispensed in community pharmacies during the 2010–2011 flu season. J Pharm Belg. 2011;4:117–121. (Article in French).
Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169.
Castilla J, Moran J, Martinez-Artola V, et al. Effectiveness of trivalent seasonal and monovalent influenza A(H1N1)2009 vaccines in population with major chronic conditions of Navarre, Spain: 2010/11 mid-season analysis. Euro Surveill. 2011;16.pii:19799.
Kissling E, Valenciano M, Cohen JM, et al. I-MOVE multi-centre case control study 2010–11: overall and stratified estimates of influenza vaccine effectiveness in Europe. PLoS One. 2011;6:e27622.
Vankerckhoven V, Van Damme P. Clinical studies assessing immunogenicity and safety of intradermally administered influenza vaccines. Expert Opin Drug Deliv. 2010;7:1109–1125.
Arnou R, Frank M, Hagel T, Prébet A. Willingness to vaccinate or get vaccinated with an intradermal seasonal influenza vaccine: a survey of general practitioners and the general public in France and Germany. Adv Ther. 2011;28:555–565.
European Commission. Commission decision of 24.2.2009 granting marketing authorisation under Regulation (EC) No 726/2004 of the European Parliament and of the Council for “INTANZA — Influenza Vaccine (split virion, inactivated)”, a medicinal product for human use. Available at: http://ec.europa.eu/health/documents/communityregister/2009/2009022454169/dec_54169_en.pdf. Accessed Jan 5 2012.
Holland D, Booy R, De Looze F, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–658.
Arnou R, Icardi G, De Decker M, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–7312.
Van Damme P, Arnou R, Kafeja F, et al. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC Infect Dis. 2010;10:134.
Reygrobellet C, Viala-Danten M, Meunier J, Weber F, Nguyen VH. Perception and acceptance of intradermal influenza vaccination: patient reported outcomes from phase 3 clinical trials. Hum Vaccin. 2010;6:336–345.
Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872.
Eizenberg P, Booy R, Naser N, et al. Acceptance of Intanza9 μg intradermal influenza vaccine in routine clinical practice in Australia and Argentina. Adv Ther. 2011;28:640–649.
Prymula R, Usluer G, Altinel S, Sichova R, Weber F. Acceptance and opinions of Intanza/IDflu intradermal influenza vaccine in the Czech Republic and Turkey. Adv Ther. 2012;29:41–52.
Organisation for Economic Co-operation and Development (OECD). OECD Health Data 2011. Health care activities — vaccination rates against influenza for people 65 and over. Available from: http://www.oecd.org/document/16/0,3746,en_2649_37407_2085200_1_1_1_37407,00.html. Accessed Jan 5 2012.
Dedoukou X, Nikolopoulos G, Maragos A, Giannoulidou S, Maltezou HC. Attitudes towards vaccination against seasonal influenza of healthcare workers in primary health-care settings in Greece. Vaccine. 2010;28:5931–5933.
Virseda S, Restrepo MA, Arranz E, et al. Seasonal and pandemic A (H1N1) 2009 influenza vaccination coverage and attitudes among healthcare workers in a Spanish University Hospital. Vaccine. 2010;28:4751–4757.
Tanguy M, Boyeau C, Pean S, Marijon E, Delhumeau A, Fanello S. Acceptance of seasonal and pandemic a (H1N1) 2009 influenza vaccination by healthcare workers in a French teaching hospital. Vaccine. 2011;29:4190–4194.
Author information
Authors and Affiliations
Corresponding author
Additional information
To view enhanced content go to www.advancesintherapy.com
Rights and permissions
About this article
Cite this article
Dhont, P.A., Albert, A., Brenders, P. et al. Acceptability of Intanza® 15 μg intradermal influenza vaccine in Belgium during the 2010–2011 influenza season. Adv Therapy 29, 562–577 (2012). https://doi.org/10.1007/s12325-012-0025-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-012-0025-9