Abstract
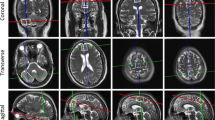
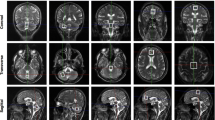
Autosomal-dominant spinocerebellar ataxia type 1 (SCA1) is an adult-onset progressive disorder with well-characterized neurodegeneration in the cerebellum and brainstem. The objective of this study is to evaluate neurochemical changes associated with neurodegeneration in cerebral tissue in SCA1 patients compared to age- and gender-matched healthy controls. Nine patients with genetically proven SCA1 and nine gender- and age-matched healthy controls were prospectively recruited from the ataxia clinic and received clinical examination. A 1.5 T single-voxel brain proton MR spectroscopy was performed for total N-acetyl aspartate (tNAA) in cerebellum, parietofrontal lobe white matter, sensory cortex, and visual cortex. In the patients, tNAA was severely decreased in the cerebellar voxel; however, in the voxels positioned in sensory cortex, parietofrontal lobe white matter and visual cortex tNAA was reduced in comparison to controls. In addition to the profoundly affected cerebellum, we also found evidence for cerebral neurodegeneration in parietal lobe white matter, sensory cortex, and visual cortex in SCA1 patients illustrating a multisystem neurodegenerative character of the disease.



Similar content being viewed by others
References
Matilla-Dueñas A, Goold R, Giunti P. Clinical, genetic, molecular, and pathophysiological insights into spinocerebellar ataxia type 1. Cerebellum. 2008;7(2):106–14.
Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9(9):885–94.
Schöls L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3(5):291–304.
Schulz JB, Borkert J, Wolf S, Schmitz-Hübsch T, Rakowicz M, Mariotti C, et al. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage. 2010;49(1):158–68.
Rüb U, Bürk K, Timmann D, den Dunnen W, Seidel K, Brunt E, et al. Spinocerebellar ataxia type 1 (SCA1): new pathoanatomical and clinico-pathological insights. Neuropathology and applied neurobiology. 2012;38(7):665–80.
Schöls L, Amoiridis G, Büttner T, Przuntek H, Epplen JT, Riess O. Autosomal dominant cerebellar ataxia: phenotypic differences in genetically defined subtypes? Ann Neurol. 1997;42(6):924–32.
Klinke I, Minnerop M, Schmitz-Hübsch T, Hendriks M, Klockgether T, Wüllner U, et al. Neuropsychological features of patients with spinocerebellar ataxia (SCA) types 1, 2, 3, and 6. Cerebellum. 2010;9(3):433–42.
Schöls L, Linnemann C, Globas C. Electrophysiology in spinocerebellar ataxias: spread of disease and characteristic findings. Cerebellum. 2008;7(2):198–203.
Ginestroni A, Della Nave R, Tessa C, Giannelli M, De Grandis D, Plasmati R, et al. Brain structural damage in spinocerebellar ataxia type 1: a VBM study. J Neurol. 2008;255(8):1153–8.
Della Nave R, Ginestroni A, Tessa C, Salvatore E, De Grandis D, Plasmati R, et al. Brain white matter damage in SCA1 and SCA2. An in vivo study using voxel-based morphometry, histogram analysis of mean diffusivity and tract-based spatial statistics. Neuroimage. 2008;43(1):10–9.
Currie S, Hadjivassiliou M, Wilkinson ID, Griffiths PD, Hoggard N. Magnetic resonance spectroscopy of the normal cerebellum: what degree of variability can be expected? Cerebellum. 2013;12(2):205–11.
Mascalchi M, Tosetti M, Plasmati R, Bianchi MC, Tessa C, Salvi F, et al. Proton magnetic resonance spectroscopy in an Italian family with spinocerebellar ataxia type 1. Ann Neurol. 1998;43(2):244–52.
Guerrini L, Lolli F, Ginestroni A, Belli G, Della Nave R, Tessa C, et al. Brainstem neurodegeneration correlates with clinical dysfunction in SCA1 but not in SCA2. A quantitative volumetric, diffusion and proton spectroscopy MR study. Brain. 2004;127(Pt 8):1785–95.
Oz G, Hutter D, Tkác I, Clark HB, Gross MD, Jiang H, et al. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord. 2010;25(9):1253–61.
Oz G, Iltis I, Hutter D, Thomas W, Bushara KO, Gomez CM. Distinct neurochemical profiles of spinocerebellar ataxias 1, 2, 6, and cerebellar multiple system atrophy. Cerebellum. 2011;10(2):208–17.
Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–20.
Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–48.
Haase A, Frahm J, Hänicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985;30(4):341–4.
Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9.
Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hänicke W, Sauter R. Localized proton NMR spectroscopy in different regions of the human brain in vivo. Relaxation times and concentrations of cerebral metabolites. Magn Reson Med. 1989;11(1):47–63.
Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30(4):424–37.
Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–53.
Lirng JF, Wang PS, Chen HC, Soong BW, Guo WY, Wu HM, et al. Differences between spinocerebellar ataxias and multiple system atrophy-cerebellar type on proton magnetic resonance spectroscopy. PLoS ONE. 2012;7(10):e47925.
Abele M, Bürk K, Andres F, Topka H, Laccone F, Bösch S, et al. Autosomal dominant cerebellar ataxia type I. Nerve conduction and evoked potential studies in families with SCA1, SCA2 and SCA3. Brain. 1997;120(Pt 12):2141–8.
Stricker S, Oberwahrenbrock T, Zimmermann H, Schroeter J, Endres M, Brandt AU, et al. Temporal retinal nerve fiber loss in patients with spinocerebellar ataxia type 1. PLoS ONE. 2011;6(7):e23024.
Reetz K, Costa AS, Mirzazade S, Lehmann A, Juzek A, Rakowicz M, et al. Genotype-specific patterns of atrophy progression are more sensitive than clinical decline in SCA1, SCA3 and SCA6. Brain. 2013;136(Pt 3):905–17.
Viau M, Marchand L, Bard C, Boulanger Y. (1)H magnetic resonance spectroscopy of autosomal ataxias. Brain Res. 2005;1049(2):191–202.
Giuffrida S, Saponara R, Restivo DA, Trovato Salinaro A, Tomarchio L, Pugliares P, et al. Supratentorial atrophy in spinocerebellar ataxia type 2: MRI study of 20 patients. J Neurol. 1999;246(5):383–8.
Brenneis C, Bösch SM, Schocke M, Wenning GK, Poewe W. Atrophy pattern in SCA2 determined by voxel-based morphometry. Neuroreport. 2003;14(14):1799–802.
Della Nave R, Ginestroni A, Tessa C, Cosottini M, Giannelli M, Salvatore E, et al. Brain structural damage in spinocerebellar ataxia type 2. A voxel-based morphometry study. Mov Disord. 2008;23(6):899–903.
Oz G, Nelson CD, Koski DM, Henry PG, Marjanska M, Deelchand DK, et al. Noninvasive detection of presymptomatic and progressive neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2010;30(10):3831–8.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG Exc. 257).
Conflicts of interest
Sarah Stricker, Alexander U Brandt, Timm Oberwahrenbrock and Jan Leo Rinnenthal report no conflict of interest. Friedemann Paul reports to have received grant support from Sanofi, Beyer, Pfizer, and Teva where he sees no direct conflict of interest with this work. Matthias Endres has no direct conflict of interests concerning this manuscript. He has received grant support from AstraZeneca and Sanofi, has participated in advisory board meetings of Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, MSD, Pfizer, Sanofi and has received honoraria from Astra Zeneca, Bayer, Berlin Chemie, Bristol-Myers Squibb, Boehringer-Ingelheim, Desitin, Eisei, Ever, Glaxo Smith Kline, MSD, Novartis, Pfizer, Sanofi, Takeda, Trommsdorff.
Author information
Authors and Affiliations
Corresponding author
Additional information
Friedemann Paul and Jan Leo Rinnenthal equally contributed to this study.
Rights and permissions
About this article
Cite this article
Doss, S., Brandt, A.U., Oberwahrenbrock, T. et al. Metabolic Evidence for Cerebral Neurodegeneration in Spinocerebellar Ataxia Type 1. Cerebellum 13, 199–206 (2014). https://doi.org/10.1007/s12311-013-0527-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-013-0527-2