Abstract
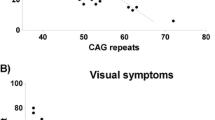
Spinocerebellar ataxia type 7 is a neurodegenerative polyglutamine disease characterized by ataxia and retinal degeneration. The longitudinal course is unknown, and relationships between repeat expansion, clinical manifestations, and neuropathology remain uncertain. We followed 16 affected individuals of a 61-member kindred over 27 years with electroretinograms, neurological examinations including the Brief Ataxia Rating Scale, neuroimaging in five, and autopsy in four cases. We identified four stages of the illness: Stage 0, gene-positive but phenotypically silent; Stage 1, no symptoms, but hyperreflexia and/or abnormal electroretinograms; Stage 2, symptoms and signs progress modestly; and Stage 3, rapid clinical progression. CAG repeat length correlated inversely with age of onset of visual or motor signs (r = −0.74, p = 0.002). Stage 3 rate of progression did not differ between cases (p = 0.18). Electroretinograms correlated with Brief Ataxia Rating Scale score and were a biomarker of disease onset and progression. All symptomatic patients developed gait ataxia, extremity dysmetria, dysarthria, dysrhythmia, and oculomotor abnormalities. Funduscopy revealed pale optic discs and pigmentary disturbances. Visual acuity declined to blindness in those with longer CAG expansions. Hyperreflexia was present from Stage 1 onwards. Restless legs syndrome and sensory impairment were common. Neuropathological hallmarks were neuronal loss in cerebellar cortex, deep cerebellar nuclei, inferior olive, and anterior horns of the spinal cord, and axonal loss in spinocerebellar tracts, dorsal nerve roots, and posterior columns. Retinal pathology included photoreceptor degeneration and disruption of retinal pigment epithelium. Spinocerebellar ataxia type 7 evolves through four clinical stages; neuropathological findings underlie the clinical presentation; electroretinograms are a potential biomarker of disease progression.






Similar content being viewed by others
References
Enevoldson TP, Sanders MD, Harding AE. Autosomal dominant cerebellar ataxia with pigmentary macular dystrophy: a clinical and genetic study of eight families. Brain. 1994;117:445–60.
David G, Durr A, Stevanin G, Cancel G, Abbas N, Benomar A, et al. Molecular and clinical correlations in autosomal dominant cerebellar ataxia with progressive macular dystrophy. Hum Mol Genet. 1998;7:165–70.
Yamada M, Sato T, Tsuji S, Takahashi H. CAG repeat disorder models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:71–86.
Helmlinger D, Hardy S, Sasorith S, Klein F, Robert F, Weber C, et al. Ataxin-7 is a subunit of GCN5 histone acetyltransferase-containing complexes. Hum Mol Genet. 2004;13:1257–65.
David G, Abbas N, Stevanin G, Durr A, Yvert G, Cancel G, et al. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat Genet. 1997;17:65–70.
Martin J, Van Regemorter N, Del-Favero J, Löfgren A, Van Broeckhoven C. Spinocerebellar ataxia type 7 (SCA7)—correlations between phenotype and genotype in one large Belgian family. J Neurol Sci. 1999;168:37–46.
To KW, Adamian M, Jakobiec FA, Berson EL. Olivopontocerebellar atrophy with retinal degeneration. An electroretinographic and histopathologic investigation. Ophthalmology. 1993;100:15–23.
Schmahmann JD, Gardner R, MacMore J, Vangel MG. Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS. Mov Disord. 2009;24:1820–8.
R Foundation for Statistical Computing [homepage on the Internet]. Vienna, Austria: Institute for Statistics and Mathematics; c1998-2011 [cited 2011 Mar 30]. R: A Language and Environment for Statistical Computing. Available from: http://www.R-project.org.
Bates DM, DebRoy S. Linear mixed models and penalized least squares. J Multivar Anal. 2004;91:1–17.
Therneau TM, Grambsch PM. Modeling survival data: Extending the Cox model. New York: Springer; 2000.
Rabin AR, Berson EL. A full-field system for clinical electroretinography. Arch Ophthalmol. 1974;92:59–63.
Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, Weigel-DiFranco C, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111:761–72.
Sandberg MA, Jacobson SG, Berson EL. Foveal cone electroretinograms in retinitis pigmentosa and juvenile macular degeneration. Am J Ophthalmol. 1979;88:702–7.
Johansson J, Forsgren L, Sandgren O, Brice A, Holmberg M. Extended CAG repeats in Swedish spinocerebellar ataxia type 7 (SCA7) patients: effect of CAG repeat length on the clinical manifestation. Hum Mol Genet. 1998;7:171–6.
Zar J. Biostatistical analysis. Englewood Cliffs: Prentice Hall; 1984.
Jacobi H, Bauer P, Giunti P, Labrum R, Sweeney MG, Charles P, et al. The natural history of spinocerebellar ataxia type 1, 2, 3, and 6: a 2-year follow-up study. Neurology. 2011;77:1035–41.
Ashizawa T, Perlman S, Gomez C, Wilmot G, Schmahmann J, Ying S, et al. Clinical characteristics of spinocerebellar ataxias 1, 2, 3, and 6. Abstract presented at: The American Academy of Neurology 65th Annual Meeting; 2012 April 24; New Orleans.
Holmberg M, Duyckaeerts C, Durr A, Cancel G, Gourfinkel-An I, Damier P, et al. Spinocerebellar ataxia type 7 (SCA7): a neurodegenerative disorder with neuronal intranuclear inclusions. Hum Mol Genet. 1998;7:913–8.
Koeppen AH. The pathogenesis of spinocerebellar ataxia. Cerebellum. 2005;4:62–73.
Neetens A, Martin JJ, Libert J, Van den Ende P. Autosomal dominant cone dystrophy-cerebellar atrophy (ADCoCA) (modified ADCA Harding II). Neuro-ophthalmol. 1990;10:261–75.
Ansorge O, Giunti P, Michalik A, Van Broeckhoven C, Harding B, Wood N, et al. Ataxin-7 aggregation and ubiquitination in infantile SCA7 with 180 CAG repeats. Ann Neurol. 2004;56:448–52.
Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–61.
Manrique RK, Noval S, Aguilar-Amat MJ, Arpa J, Rosa I, Contreras I. Ophthalmic features of spinocerebellar ataxia type 7. J Neuroophthalmol. 2009;29:174–9.
Miller RC, Tewari A, Miller JA, Garbern J, Van Stavern GP. Neuro-ophthalmologic features of spinocerebellar ataxia type 7. J Neuroophthalmol. 2009;29:180–6.
Oh AK, Jacobson KM, Jen JC, Baloh RW. Slowing of voluntary and involuntary saccades: an early sign in spinocerebellar ataxia type 7. Ann Neurol. 2001;49:801–4.
Martin J, Van Regemorter N, Krols L, Brucher JM, de Barsy T, Szliwowski H, et al. On an autosomal dominant form of retinal-cerebellar degeneration: an autopsy study of five patients in one family. Acta Neuropathol. 1994;88:277–86.
Kedia S, Moro E, Tagliati M, Lang AE, Kumar R. Emergence of restless legs syndrome during subthalamic stimulation for Parkinson disease. Neurology. 2004;63:2410–2.
Driver-Dunckley E, Evidente VGH, Adler CH, Hillman R, Hernandez J, Fletcher G, et al. Restless legs syndrome in Parkinson's disease patients may improve with subthalamic stimulation. Mov Disord. 2006;21:1287–9.
Elden AC, Kim H, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, et al. Ataxin-2 intermediate-length polyglutamine expanions are associated with increased risk for ALS. Nature. 2010;466:1069–75.
Holmes G. The cerebellum of man. Brain. 1939;62:1–30.
Brown P. Pathophysiology of spasticity. J Neurol Neurosurg Psychiatry. 1994;57:773–7.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79.
Berson EL. Long-term visual prognoses in patients with retinitis pigmentosa: the Ludwig von Sallmann lecture. Exp Eye Res. 2007;85:7–14.
Acknowledgements
Our paper is dedicated to the members of this family, whose personal resolve and commitment to this study are inspirational. Dr. Katherine B. Sims performed the original genetic testing in this family. The assistance of Jason MacMore is gratefully acknowledged.
Funding
This work was supported by the MINDlink Foundation, the Birmingham Foundation, the Foundation Fighting Blindness, and the Massachusetts Alzheimer Disease Research Center [AG05134].
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
II-3; 45 CAG repeats
At age 57, II-3 noticed unsteady gait. This progressed slowly until age 67, when she noted more rapid decline with worsening gait, slurred speech, and occasional choking. At age 69, she was diagnosed with macular degeneration.
At age 70, acuity was 20/50 OU. Eye movements were slowed, and there were saccadic intrusions into pursuit and with testing for vestibulo-ocular reflex cancellation (VORC). Speech was mildly dysarthric. Finger-to-nose and heel-to-shin testing revealed dysmetria. BARS was 12. Reflexes were brisk throughout with clonus at the left ankle. Plantar responses were extensor bilaterally. Pin appreciation was diminished distally. Romberg test was positive.
At age 71, she reported central visual field loss, urinary urge incontinence, and RLS, which responded to carbidopa-levodopa. Crossed-supraclavicular and crossed-pectoral reflexes were noted.
Over the following 9 years, her condition continued to deteriorate. She reported “terrible shakes.” Leg spasms responded to baclofen. She could walk only with assistance from at least one person.
At age 80, central visual loss, ataxia, dysmetria, dysarthria, and ophthalmoparesis were severe. She could take a few steps with substantial, two-person assistance. BARS was 25.5. She had buccofacial and lingual dyskinesias. She reported complex, non-threatening visual hallucinations (Charles Bonnet syndrome) precipitated by use of amantadine. Limited MRI images showed mild generalized volume loss, more prominent in the cerebellum (Fig. 3).
She died at 81, and autopsy was performed.
II-5; 46 CAG repeats
Gait problems commenced at age 55. He fractured the left hip at 64 and began using a wheelchair. Loss of visual acuity was noted at 65; he was legally blind by 67. He reported that his legs would “jump like crazy” during the day and night.
At age 67, visual acuity was 20/400 bilaterally, and the optic discs were pale. Eye movements were slowed with saccadic intrusions noted during pursuit and VORC testing. Pupils reacted slowly. High frequency hearing was decreased on the left. He was mildly dysarthric. Tone was increased throughout with mild spastic catch in the lower extremities. Finger-to-nose and heel-to-shin testing revealed dysmetria. Gait was wide-based, stiff-legged, and unsteady. BARS was 13. Deep tendon reflexes (DTRs) were graded 3 throughout, and 4 at the ankles. Plantar responses were extensor. Position sense was impaired. He was disinhibited. Semantic fluency was markedly decreased to five animals in 1 min. The Similarities test revealed failure of complex reasoning.
He died age 73, and autopsy was performed.
III-6; 46 CAG repeats
III-6 was diagnosed with retinitis pigmentosa at the age of 37. At age 47, he had difficulty balancing on skates. His gait became stiff-legged. While he had always been partially colorblind, his vision started to decline at this time.
At age 49, vision was 20/50 bilaterally with increased pigment around the maculae. Pursuit eye movements and saccades were slowed. There was saccadic breakdown of the vestibulo-ocular reflex cancellation (VORC). Blinking and eye shifts were slowed. There was a moderate degree of dysarthria. Tone was increased with a spastic catch bilaterally in the upper and lower extremities, with clonus in the left upper extremity. Rapid foot tapping was severely abnormal bilaterally. There was moderate end-point dysmetria on the finger-to-nose test and decomposition of movement on the heel-to-shin test. Gait was unsteady, and he was unable to perform tandem gait. BARS was 9 out of 30. Deep tendon reflexes (DTRs) were graded 3 in the upper and lower extremities and 4 at the ankles; plantar responses were extensor bilaterally. Hoffmann's sign and crossed-pectoral and crossed-adductor reflexes were present bilaterally. Jaw jerk and palmar grasp were negative. Vibration sense was decreased at the toes and ankles.
Electroretinography performed at age 51 showed retinal malfunction. He had restricted ocular movements, failure in color testing, and computerized acuity mapping of the macula was abnormal.
At 53, he reported slowing down in his overall functioning. He required two rails to ascend stairs. Handwriting had worsened. BARS was 8.
At 58, he had nocturnal myoclonus and suffered a major fall due to retropulsion. Optic discs were pale bilaterally. Pursuit eye movements were markedly slowed. BARS was 12. DTRs remained exaggerated, now with spread from the biceps and brachioradialis to the hands.
At the age of 60, he had occasional falls and choking, and urinary urgency. He was unable to walk independently without holding onto a wall, and started using a walker. BARS was 14.
At 61, visual acuity was 20/70 bilaterally. BARS was 15. Six months later, BARS was 17.5.
At 62, he was legally blind, and visual acuity was 20/200 bilaterally. BARS was 18.
III-7; 41 CAG repeats
III-7 developed gait impairment at age 42. At age 46, she had untidy handwriting, impaired articulation, and mild problems with short-term memory. Examination at age 48 revealed end-gaze nystagmus, saccadic intrusions into the vestibulo-ocular reflex cancellation (VORC) test, and a subtle, lingual-predominant cerebellar dysarthria. Tone was decreased with a spastic catch in the upper extremities. There was subtle dysrhythmia with rapid tapping of the feet and subtle dysmetria with the finger-to-nose test. Stress gait elicited posturing of both hands. BARS was 3. She was hyperreflexic in all extremities, with positive Hoffmann's signs and crossed-adductor and crossed-supraclavicular reflexes bilaterally. Plantar responses were silent.
At age 51, pursuit eye movements were slowed with overshoot on saccades. Gait was wide-based and mildly unsteady. BARS was 8. There was sustained bilateral ankle clonus.
At age 60, she reported a “nosedive” with faster deterioration of handwriting, balance, and speech. A sleep study revealed RLS. She had occasional urinary urgency. Rapid alternating syllables were impaired. BARS was 6.5; this improved from age 51 due to absence of side-to-side dysmetria on the heel-to-shin test. Phonemic fluency was reduced to five F-words in a minute.
III-8; 41 CAG repeats
At age 46, III-8 felt asymptomatic. Upon examination, there was subtle saccadic intrusion into VORC. Upper extremities had a spastic catch, with mildly increased tone in the right upper extremity. On a finger-to-nose test, the left hand was slightly slower and produced mirror movements in the right hand. Heel-to-shin testing was decomposed into several phases in-plane, with proximal overshoot bilaterally. BARS was 3. DTRs were graded 2+ throughout with 3 beats of clonus at the ankles. Crossed-supraclavicular and crossed-adductor reflexes were subtly present on the left. Plantar responses were bilaterally flexor.
At age 48, DTRs were graded 3 throughout, with 4 beats of clonus in the ankles. Stress gait produced cortical thumb on the left hand and internal posturing of the right hand. BARS remained 3.
Clinical signs remained otherwise stable until age 52, when his gait was slightly wide-based. Handwriting had worsened. His wife reported memory decline. Speech was irregular with alternating syllables. The heel-to-shin test was subtly impaired on the left, and there was some breakdown of pronation and supination with the left hand. BARS was 1.5. DTRs were graded 3 throughout, but 4 at the left ankle with 6 beats of clonus.
At age 55, there was unsteadiness while descending stairs and choking if he ate too quickly. Saccades were slowed. There was mild overshoot in both upper extremities and some irregularity with rapid pronating and supinating movements of the left arm, which produced mirror movements on the right. BARS score was 3.5. The left plantar response was now extensor.
At age 56, he reported sleep disturbances due to leg movements and tingling. He was choking about twice a week while eating. Clinical signs were otherwise unchanged.
At 57, carbidopa-levodopa helped diminish his restless legs syndrome. There was mild end-point dysmetria in the right upper extremity on the finger-to-nose test. Tandem gait was impaired. BARS score was 5.
At age 58, he discontinued use of carbidopa-levodopa and reported that “soap in the bed” effectively treated his restless legs syndrome. He was unable to descend steps without using the handrail, and had to hold on to his wife. End-point dysmetria on the finger-to-nose test was now evident bilaterally. Dysarthria was mild. He required an extra step on tandem gait. BARS was 7. Hoffman's and crossed-supraclavicular and crossed-pectoral reflexes were present.
III-12; 41 CAG repeats
III-12 had no history of balance or gait impairment when first seen at age 34. She reported occasional numbness and heaviness in the left calf. Neurological examination was normal, with the exception of minimal right hand posturing during stress gait and a slight decrease in pin appreciation between the foot and the thigh. BARS was 0.
At age 36, rapid foot tapping was dysrhythmic. BARS remained 0. DTRs were now diffusely exaggerated.
At age 46, BARS remained 0. Examination revealed only hyperreflexia throughout including positive Hoffman, and ipsilateral, crossed-supraclavicular, and pectoral reflexes, but negative jaw jerk.
III-13; 47 CAG repeats
At age 40, family members of III-13 noticed abnormalities in her gait and slurring in her speech, saying she seemed “drunk.” She had double vision at age 45 that worsened with fatigue. At age 48, she began using a cane, and noticed more rapid deterioration in her gait. At age 49, she reported that her handwriting was no longer legible. She noted a decline in memory, reporting difficulty completing crossword puzzles. Her legs “would jump on their own,” especially when placing the ball of the foot on the ground. She had dizziness when standing from a sitting position.
At age 49, pursuit eye movements and saccades were slowed without hypermetria. There was a lag in VORC and decreased spontaneous eye movements with a mild stare. Tone was increased in the upper extremities with a spastic catch, left more than right. Tone in the lower extremities was normal with a mild spastic catch. Rapid foot tapping was slow and dysrhythmic. The finger-to-nose test was slow with mild terminal dysmetria; rapid alternating movements were slow and awkward bilaterally, and there was overshoot on rapid finger following. BARS was 9. DTRs were graded 3 in the upper extremities and 4 in the legs with sustained clonus at the knees and ankles bilaterally. Hoffmann's and Wartenberg's signs were positive. Crossed-pectoral, crossed-supraclavicular, and crossed-adductor reflexes were present; plantar responses were extensor bilaterally. Jaw jerk was absent. Forward digit span was 7 with 1 transposition, and reverse digit span was impaired at 2. Semantic fluency was impaired at 14 animals in a minute.
At age 54, she reported difficulty swallowing, with frequent choking spells. There was optic atrophy bilaterally. She had poor color vision and could not read large print on book covers. There was exophoria of the left eye. Tone was markedly increased in the lower extremities. Gait was essentially impossible without support on both sides; the legs were stiff, spastic, and dysmetric. BARS was 14. Vibration sense was decreased in the feet, and pin appreciation was decreased distally in the upper and lower extremities.
A feeding tube was placed at age 58. Vision began declining more noticeably at age 59. At age 61, she reported that her right leg had begun kicking out on its own while sitting on the chair in the shower. Pupils reacted from 7 to 6 mm on the right and from 6 to 5 mm on the left eye. Upgaze was limited to approximately 5°. Striking optic atrophy was present bilaterally. Pursuit and saccadic eye movements were severely slowed. Speech was moderately to severely slow with mild to moderate cerebellar dysarthria. A spastic catch was present in the upper and lower extremities. Strength was full in the upper extremities and slightly decreased in the lower extremities, graded 4+ proximally and distally. Finger-to-nose testing was markedly impaired. There was side-to-side dysmetria on heel-to-shin testing. She was confined to a wheelchair. BARS was 24.5. DTRs were 3+ in the upper extremities, with sustained clonus at the knees and ankles and clonus with finger flexion. Appreciation of cold temperature was absent at the calves. On mental state testing, she was unsure of the precise date and could visualize the President but only approximate his name. She learned four words on the first attempt and recalled three in 5 min; she was unable to recall the fourth word with multiple choice. Digit span was 5 forward and 2 in reverse. Verbal fluency was markedly decreased with semantic fluency of five animals and phonemic fluency of four F-words.
III-15; 41 CAG repeats
At age 46, III-15 was asymptomatic. Neurological examination including mental state testing was normal. BARS was 0.
III-16; 55 CAG repeats
III-16 first noticed a decline in visual acuity at age 16; corrective lenses were helpful until age 21.
At age 22, neurological examination performed elsewhere was reported to be normal apart from hyperreflexia throughout with 3 beats of clonus at the ankles. The corresponding BARS score was 0.
By age 28, he was able to read only large newsprint. Optic discs were pale with granular macular degeneration. Visual acuity was 20/100 bilaterally. Pupils responded sluggishly. Pursuit eye movements were slow with upgaze. Gaze-evoked, direction-beating nystagmus was present with upward and lateral gaze. Speech was dysarthric. Tone was increased in the lower extremities. Finger-to-nose and heel-to-shin testing was dysmetric. Gait was wide-based and ataxic. BARS was 13.5. DTRs were exaggerated, with 3–4 beats of clonus at the ankles. Plantar responses were now extensor. Romberg test was positive.
His condition worsened over the next 10 years. By 38, he was unable to walk or push his wheelchair secondary to vision loss. He was constantly dizzy, and vision was blurred. A sleep study showed severely increased periodic limb movements of sleep and frequent limb-associated arousal. Dysarthria, hypertonia, dysmetria, and dysphagia were severe.
At 42, he was wheelchair-bound. He reported frequent choking and RLS. He had no light perception, and pupils were nonreactive. There was complete external ophthalmoplegia. There were tongue fasciculations and frequent facial grimaces. Finger-to-nose and heel-to-shin testing could not be performed. He developed contractures in the upper extremities, became unable to move his lower extremities, which were held in extension, and dysarthria was profound. BARS was 29.5, and he died age 43 of bronchopneumonia, and autopsy was performed.
IV-19; CAG unknown, but suspected >100
IV-19 began walking at 12 months. At age 2.5, his parents noticed tremor in the hands, which improved transiently with propranolol and mysoline. His running became irregular at age 3.5. When examined at age 3 years 10 months, speech was slurred and frenetic. He was “very jittery” and unable to hop on one foot (David et al. [2]).
At 4 years 2 months, he fell frequently and was incontinent. Head circumference was at the fifth percentile for age, and weight was at the 25th percentile. He had bilateral esotropia with poor vision, and prominent dysarthria and ataxia. Tone and DTRs were decreased. Ophthalmologic examination showed a small area of hypopigmentation in the midperipheral fundus of the left eye.
He had an apnea episode at 4 years 11 months. At 5 years old, he had retinitis pigmentosa and was legally blind. Funduscopic examination showed narrowing of retinal arterioles and granularity in the peripheral retina of both eyes. He was weak throughout. A gastric tube was placed.
At age 6, there was complete loss of vision, extraocular motion, and speech. He died at 6 years and 2 months, and autopsy was performed.
IV-20; 44 CAG repeats
IV-20 was healthy until her early twenties, when she noticed that she was clumsy and off-balance. She started using glasses at age 23, when she noticed progressive decline in vision. She was diagnosed with Graves' disease at age 25. She gave up driving at age 32, due to visual loss. Handwriting and fine dexterity were affected, and balance was unsteady. Restrictions of daily activities and deaths of family members caused isolation, anxiety, and depression; she felt “off her rocker.”
At age 33, pursuit eye movements and saccades were slowed, and visual acuity was 20/200. She had mild dysarthria. There was mild dysmetria and decomposition of movement on the finger-to-nose test. The heel-to-shin test showed jerking in-plane, worse on the left lower extremity, and slowing in phases on the right. Gait was mildly impaired and wide-based with an occasional step to the side; she could not perform tandem gait. BARS was 9. DTRs were graded 3 throughout with spread from the brachioradialis to the fingers. Crossed-pectoral and crossed-adductor reflexes were present but subtle. Plantar responses were silent bilaterally.
IV-22; 46 CAG repeats
IV-22 noticed unsteadiness while walking and climbing stairs at age 25. At age 27, he was mildly dysarthric. Tone was slightly increased in the lower extremities. Fine motor movements were mildly dysmetric bilaterally. Gait was unstable with a wide base, and he could not perform tandem gait. BARS was 3.5. Deep tendon reflexes were graded 3+ throughout with 2–3 beats of ankle clonus. The left plantar response was flexor, while the right was possibly extensor.
At age 29, he reported progression of his balance problems and slurring of speech. He noted recently having difficulty reading words on the television screen. Saccadic eye movements were slowed. Speech was dysarthric. Tone was mildly increased in the upper extremities and left lower extremity. There was mild bradykinesia on repetitive movements of the upper and lower extremities. His gait showed a 3-in. base with diminished arm swing. BARS was 6.5. DTRs were 3+ throughout, mildly pendular at the knees and with 3–4 beats of clonus at the ankles. Plantar responses were flexor bilaterally.
At age 33, he reported feeling worse overall. He was experiencing choking when swallowing liquids and solids. Neurological examination revealed saccadic smooth pursuit and very slow saccades. Bulk and tone were normal in the upper extremities, and the lower extremities were very spastic. There was mild ataxia on finger-to-nose testing bilaterally. Rapid alternating movements and finger tapping were slowed bilaterally. There was no significant ataxia noted on heel-to-shin testing. BARS was 10. DTRs were very brisk throughout, with 4 beats of clonus in the right ankle and 7 beats in the left ankle.
IV-36; 51 CAG repeats
IV-36 was diagnosed with degenerative retinal disease at age 18, and began to experience balance difficulties at age 25. She suffered from migraines since the age of 12. When examined at 28, she reported seeing flashes at the bottom of the visual field. The optic discs were pale, and pursuit eye movements were slow. She had gaze-evoked direction-beating nystagmus on upgaze, and decreased spontaneous eye movements. There were small involuntary movements of the tongue. Dysarthria was spastic and ataxic. Tone was increased in all extremities, left more than right. Finger-to-nose movements were slowed mildly on the right upper extremity and moderately on the left. With the heel-to-shin test, there was proximal overshoot and minimal side-to-side dysmetria. Gait was spastic, slow, and unsteady; she was unable to perform tandem gait. BARS was 12.5. DTRs were exaggerated with clonus throughout. Hoffmann's and Wartenberg's signs, jaw jerk, and crossed-supraclavicular and crossed-adductor reflexes were all present. Vibration sense was decreased at the toes. Pin appreciation was decreased below mid-calf. Semantic fluency was reduced to 14 animals in a minute; serial 7 subtraction was impaired, and complex similarities were concrete.
At age 37, she reported crying for no obvious reason, having difficulty mixing up words, particularly pronouns, being confused about the month, forgetting her sentences midstream, and repeating herself in conversation. She had urinary incontinence, daily headaches, and aching pain associated with nausea, which was worsened by bright lights. Vision had deteriorated: she had only a vague sense of outlines and shapes. She reported weakness in the upper extremities, left more than right, and numbness in the feet. She was legally blind, but still maintained some light perception. She was confined to a wheelchair, and gait was impossible even with assistance. BARS was 21. DTRs were exaggerated throughout, with clonus at the ankles and knees. Hoffmann's sign was positive with clonus bilaterally. Jaw jerk was negative. Vibration sense was diminished at the toes; position sense was absent at the toes and ankles. Pin appreciation was absent from the mid-thighs distally and from the elbows distally.
At the age of 39, she was completely blind.
At age 40, she scalded her perineum and feet from hot water in the bath that she was unable to turn off, and required nursing home care. Food intake required the use of nectar, and she reported coughing on saliva approximately once a week. Eye movements showed less than 10° of movement in the horizontal direction, with no upward gaze, and 5° of downward gaze. Pursuit eye movements and saccades were severely slowed. Pupils were nonreactive to light bilaterally, and there was no light perception. Funduscopic examination revealed prominent pallor of the optic discs and pigmentary change of the retinas bilaterally. There were intermittent facial dyskinesias with grimacing around the face and occasionally the eyes. Legs were held in extension, and passive flexion was not possible. There was a prominent spastic catch in the upper extremities. Bulk was mildly decreased throughout. Strength was mildly decreased throughout. Adapted finger-to-nose testing revealed slowed movements with breakdown at the elbow. Attempts at rapid alternating movements were severely slowed. Heel-to-shin testing showed in-plane jerking. BARS was 22.5. There was dramatic hyperreflexia, graded 3+ in the upper extremities and right knee, 4 at the left knee with patellar clonus, and 4 at both ankles with sustained clonus. Plantar responses were extensor bilaterally. On mental state testing, she was unable to subtract serial 7s. She was markedly depressed.
IV-37; 49 CAG repeats
At age 26, IV-37 was asymptomatic. There were saccadic intrusions into VORC and 2 beats of gaze-evoked direction beating nystagmus in the horizontal plane. There was a spastic catch in the upper extremities bilaterally. Finger-to-nose testing showed subtle end point dysmetria and overshoot with rapid finger following. Rapid alternating movements were slowed and awkward bilaterally. There was mild proximal overshoot and subtle in-plane jerking on the heel-to-shin test. BARS was 5. DTRs were exaggerated with 4 beats of clonus at the ankles bilaterally. Hoffmann's sign and crossed-adductor and crossed-supraclavicular reflexes were present.
He stopped running at age 35 and noticed problems with balance at age 36. He became aware of his own hyperreflexia. At age 38, the optic discs exhibited bilateral temporal pallor with no abnormal retinal pigmentation. Saccades were slightly slowed. There was mild dysrhythmia on alternating syllables. There were spastic catches in the upper and lower extremities bilaterally, right more than left. Finger-to-nose testing revealed mild tremor at the endpoint. Mild bilateral in-plane jerking was observed on heel-to-shin testing. His gait had a widened stance and occasional veering. He could not perform tandem gait. There was truncal titubation when standing with feet together. BARS was 9. DTRs were graded 3+ in the arms and 4 in the legs with sustained clonus at the ankles bilaterally and patellar clonus at the knees. Plantar responses were flexor. There was clonus at finger flexion, and bilateral Hoffmann and crossed-adductor, crossed-supraclavicular, and pectoral reflexes were present. The jaw jerk was negative.
IV-43; 63 CAG repeats
IV-43 was born prematurely. At 1 year of age, weight was below the third percentile. At 12, he would fall easily. CT and MRI were performed, and he was diagnosed with olivopontocerebellar atrophy. By age 14, he required a cane to walk. At age 15, speech was difficult to comprehend, and he began using a wheelchair. Vision began declining at 16.
At age 17, he was able to perceive light but could barely count fingers. Optic discs were pale bilaterally; maculae showed patchy depigmentation with scattered spicules. Eye movements were severely slowed. Pupils reacted minimally to light. Speech was dysarthric. Tone was markedly increased throughout, and there was clonus in all extremities with attempted straightening. Finger-to-nose and heel-to-shin testing was dysmetric. He was able to walk with support; gait was spastic, ataxic, high-stepping, and wide-based. BARS was 22.5. DTRs were brisk; plantar responses were extensor. Position sense was decreased in the toes. Semantic fluency was reduced to 11 animals in a minute. He was unable to provide complex similarities.
At age 19, he was cachectic. Spontaneous facial grimacing and fasciculations of the cheek were notable. Speech was profoundly dysarthric. Finger-to-nose and heel-to-shin testing could not be performed. BARS was 28.
At age 20, he had total external ophthalmoplegia. There was prominent bruxism. At age 21, he was completely blind, and pupils were nonreactive. BARS was 29.5. Vibration sense was impaired in the legs.
He died at 21.
Rights and permissions
About this article
Cite this article
Horton, L.C., Frosch, M.P., Vangel, M.G. et al. Spinocerebellar Ataxia Type 7: Clinical Course, Phenotype–Genotype Correlations, and Neuropathology. Cerebellum 12, 176–193 (2013). https://doi.org/10.1007/s12311-012-0412-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-012-0412-4