Abstract
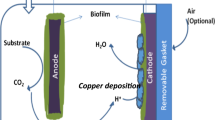
We investigate the workability of electron transfer mediators (ETMs) for the enhanced microbial fuel cell (MFC) operated by Shewanella putrefaciens CN32. In open-circuit, natural ETMs showed 128 ~ 166% of enhancement in the difference of electrical potential compared to that without ETMs (0.41 V), while MFC with synthetic ETMs achieved 200 ~ 250% of enhancement, showing a liner relationship between the electric potentials and ETMs’ redox potentials (R2 = 0.91). Especially, flavin mononucleotide (FMN), the most effective ETM for Shewanella-MFC operation, exhibited the highest potential (0.547 V) and power density (20.28 mW/m2) and generated the maximum current (0.285 mA). The addition of conductive iron-bearing minerals (hematite: 26.27 mW/m2, lepidocrocite: 25.83 mW/m2) enhanced power density of FMN-MFC, while no significant enhancement was observed when soluble iron source (ferric citrate) was added. In addition, MFC containing hematite showed the maximum current (0.37 mA) with 30.8 % of coulombic efficiency.
Similar content being viewed by others
References
Logan, B. E. and J. M. Regan (2006) Electricity-producing bacterial communities in microbial fuel cells. Trends Microbial. 14: 512–518.
Kim, H. J., H. S. Park, M. S. Hyun, I. S. Chang, M. Kim and B. H. Kim (2002) A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enz. Microb. Tech. 30: 145–152.
Min, B., S. Cheng, and B. E. Logan (2005) Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 39: 1675–1686.
Liu, Z. D., J. Lian, Z. W. Du, and H. R. Li (2006) Construction of sugar-based microbial fuel cells by dissimilatory metal reduction bacteria. Chin. J. Biotechnol. 22: 131–137.
Liu, Z. D., Z. W. Du, J. Lian, X. Y. Zhu, S. H. Li, and H. R. Li (2007) Improving energy accumulation of microbial fuel cells by metabolism regulation using Rhodoferax ferrireducens as biocatalyst. Lett. Appl. Microbiol. 44: 393–398.
Du, Z., H. Li, and T. Gu (2007) A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 25: 464–482.
Logan, B. E. (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 7: 375–381.
Kim, B. H., T. Ikeda, H. S. Park, H. J. Kim, M. S. Hyun, K. Kano, K. Takagi, and H. Tatsumi (1999) Electrochemical activity of an Fe (III)-reducing bacterium, Shewanella putrefaciens IR-1, in the presence of alternative electron acceptors. Biotechnol. Tech. 13: 475–478.
Kim, H. J., M. S. Hyun, I. S. Chang, and B. H. Kim (1999) A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9: 365–367.
Myers, C. R. and J. M. Myers (1992) Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Bacteriol. 174: 3429–3438.
El-Naggar, M. Y., G. Wanger, K. M. Leung, T. D. Yuzvinsky, G. Southam, J. Yang, W. M. Lau, K. H. Nealson, and Y. A. Gorby (2010) Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc. Natl. Acad. Sci. USA. 107: 18127–18131.
Wu, C. Y., L. Zhuang, S. G. Zhou, Y. Yuan, T. Yuan, and F. B. Li (2012) Humic substance-mediated reduction of iron (III) oxides and degradation of 2, 4-D by an alkaliphilic bacterium, Corynebacterium humireducens MFC-5. Microb. Biotechnol. 6: 141–149.
Aeschbacher, M., D. Vergari, R. P. Schwarzenbach, and M. Sander (2011) Electrochemical analysis of proton and electron transfer equilibria of the reducible moieties in humic acids. Environ. Sci. Technol. 45: 8385–8394.
Sun, J., W. Li, Y. Li, Y. Hu, and Y. Zhang (2013) Redox mediator enhanced simultaneous decolorization of azo dye and bioelectricity generation in air-cathode microbial fuel cell. Bioresour. Technol. 142: 407–414.
Park, D. and J. Zeikus (2002) Impact of electrode composition on electricity generation in a single-compartment fuel cell using Shewanella putrefaciens. Appl. Microbiol. Biot. 59: 58–61.
Ringeisen, B. R., E. Henderson, P. K. Wu, J. Pietron, R. Ray, B. Little, J. C. Biffinger, and J. M. Jones-Meehan (2006) High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ. Sci. Technol. 40: 2629–2634.
Feng, C., L. Ma, F. Li, H. Mai, X. Lang, and S. Fan (2010) A polypyrrole/anthraquinone-2, 6-disulphonic disodium salt (PPy/AQDS)-modified anode to improve performance of microbial fuel cells. Biosens. Bioelectron. 25: 1516–1520.
Bae, S. and W. Lee (2013) Biotransformation of lepidocrocite in the presence of quinones and flavins. Geochim. Cosmochim. Acta. 114: 144–155.
Bae, S. and W. Lee (2014) Influence of riboflavin on nanoscale zero-valent iron reactivity during the degradation of carbon tetrachloride. Environ. Sci. Technol. 48: 2368–2376.
Bae, S., Y. Lee, M. J. Kwon, and W. Lee (2014) Riboflavinmediated RDX transformation in the presence of Shewanella putrefaciens CN32 and lepidocrocite. J. Hazard. Mater. 274: 24–31.
Marsili, E., D. B. Baron, I. D. Shikhare, D. Coursolle, J. A. Gralnick, and D. R. Bond (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA. 105: 3968–3973.
Von Canstein, H., J. Ogawa, S. Shimizu, and J. R. Lloyd (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microb. 74: 615–623.
Anderson, R. F. (1983) Energetics of the one-electron reduction steps of riboflavin, FMN and FAD to their fully reduced forms. BBA-Bioenergetics 722: 158–162.
Heering, H. A. and W. R. Hagen (1996) Complex electrochemistry of flavodoxin at carbon-based electrodes: results from a combination of direct electron transfer, flavin-mediated electron transfer and comproportionation. J. Electroanal. Chem. 404: 249–260.
Yamashita, M., S. S. Rosatto, and L. T. Kubota (2002) Electrochemical comparative study of riboflavin, FMN and FAD immobilized on the silica gel modified with zirconium oxide. J. Brazil Chem. Soc. 13: 635–641.
Garjonyte, R., A. Malinauskas, and L. Gorton (2003) Investigation of electrochemical properties of FMN and FAD adsorbed on titanium electrode. Bioelectrochemi. 61: 39–49.
Scott, D. T., D. M. McKnight, E. L. Blunt-Harris, S. E. Kolesar, and D. R. Lovley (1998) Quinone moieties act as electron acceptors in the reduction of humic substances by humicsreducing microorganisms. Environ. Sci. Technol. 32: 2984–2989.
Nevin, K. P. and D. R Lovley (2002) Mechanisms for Fe (III) oxide reduction in sedimentary environments. Geomicrobiol. J. 19: 141–159.
Pandit, S., S. Khilari, S. Roy, D. Pradhan, and D. Das (2014) Improvement of power generation using Shewanella putrefaciens mediated bioanode in a single chambered Microbial Fuel Cell: Effect of different anodic operating conditions. Bioresour. Technol. 166: 451–457.
Mayhew, S. G. (1999) The effects of pH and semiquinone formation on the oxidation–reduction potentials of flavin mononucleotide. Eur. J. Biochem. 265: 698–702.
Bird, L. J., V. Bonnefoy, and D. K. Newman (2011) Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol. 19: 330–340.
Kato, S., K. Hashimoto, and K. Watanabe (2012) Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA. 109: 10042–10046.
Nakamura, R., F. Kai, A. Okamoto, G. J. Newton, and K. Hashimoto (2009) Self-constructed electrically conductive bacterial networks. Angew. Chem. Int. Edit. 48: 508–511.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, Y., Bae, S., Moon, C. et al. Flavin mononucleotide mediated microbial fuel cell in the presence of Shewanella putrefaciens CN32 and iron-bearing mineral. Biotechnol Bioproc E 20, 894–900 (2015). https://doi.org/10.1007/s12257-015-0031-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-015-0031-2