Abstract
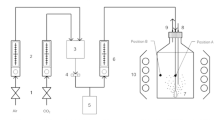
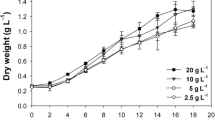
The objective of this study was to estimate the CO2 absorptivity provided by an in situ carbon supply system using a photosynthetic culture of the cyanobacterium Spirulina platensis in an open raceway pond. The effects of initial total carbon concentrations (ranging from 0 to 0.1 mol/L), suspension depths (ranging from 5 to 20 cm) and pH values (ranging from 8.9 to 11.0) on the CO2 absorptivity were studied. The results indicated that CO2 absorptivity was positively correlated with pH value, negatively correlated with total carbon concentration, and only negligibly affected by the suspension depth. The optimum total carbon concentration range and pH range were 0.03 ∼ 0.09 mol/L and 9.7 ∼ 10.0, respectively. An average CO2 absorptivity of 86.16% and average CO2 utilization efficiency of 79.18% were achieved using this in situ carbon-supply system in large-scale cultivation of Spirulina platensis, with an initial total carbon concentration of 0.06 mol/L and pH value of 9.8. Our results demonstrated that this system could obtain a favorable CO2 utilization efficiency in outdoor, large-scale cultivation of Spirulina platensis in open raceway ponds.
Similar content being viewed by others
References
Gordillo, F. J. L., C. Jiménez, F. L. Figueroa, and F. X. Niell (1998) Effects of increased atmospheric CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira). J. Appl. Phycol. 10: 461–469.
Qiang, H., H. Guterman, and A. Richmond (1996) Physiological characteristics of Spirulina plantensis (cyanobacteria) cultured at ultrahigh cell densities. J. Phycol. 32: 1066–1073.
Soletto, D., L. Binaghi, L. Ferrari, A. Lodi, J. Carvalho, M. Zilli, and A. Converti (2008) Effects of carbon dioxide feeding rate and light intensity on the fed-batch pulse-feeding cultivation of Spirulina platensis in helical photobioreactor. Biochem. Eng. J. 39: 369–375.
Vonshak, A., L. Chanawongse, B. Bunnag, and M. Tanticharoen (1996a) Light acclimation and photoinhibition in three Spirulina plantensis (cyanobacteria) isolates. J. Appl. Phycol. 8: 35–40.
Vonshak, A., N. Kancharaksa, B. Bunnag, and M. Tanticharoen (1996b) Role of light and photosynthesis on the acclimation process of the cyanobacterium spirulina platensis to salinity stress. J. Appl. Phycol. 8: 119–124.
Becker, E. W. (1994) Microalgae biotechnology and microbiology. pp. 63–171. Cambridge University Press, Cambridge, UK.
Richmond, A. and E. Becker (1986) Technological aspects of mass cultivation, a general outline. pp. 245–253. In: Richmond, A. (ed.) Handbook of microalgal mass culture. CRC Press Inc, Florida, USA.
Moazami, N., R. Ranjbar, A. Ashori, M. Tangestani, and A. Sheykhi Nejad (2011) Biomass and lipid productivities of marine microalgae isolated from the Persian Gulf and the Qeshm Island. Biomass Bioenerg. 35: 1935–1939.
Borowrrzka, M. A. (2005) Culturing microalgae in outdoor ponds. pp. 205–206. In: Andersen, R. A. (ed.) Algal culturing techniques. Academic Press, NY, USA.
Jiménez, C., B. R. Cossío, and F. X. Niell (2003) Relationship between physicochemical variables and productivity in open ponds for the production of Spirulina: A predictive model of algal yield. Aquaculture 221: 331–345.
Morais, M. G. and J. A. V. Costa (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 129: 419–425.
Binaghi, L., A. D. Borghi, A. Lodi, A. Converti, and M. D. Borghi (2003) Batch and fed-batch uptake of carbon dioxide by Spirulina platensis. Proc. Biochem. 38: 1341–1346.
Hase, R., H. Oikawa, C. Sasao, M. Morita, and Y. Watanabe (2000) Photosynthetic production of microalgal biomass in a raceway system under greenhouse condition in Sendai city. J. Biosci. Bioeng. 89: 157–163.
Cornet, J. F., C. G. Dussap, and J. B. Gros (1998) Kinetics and energetics of photosynthetic micro-organisms in photobioreactors: Application to Spirulina growth. Adv. Biochem. Eng. Biotechnol. 59: 155–224.
Cong, W., Z. F. Su, R. J. Kang, C. Y. Yang, and Z. L. Cai (2007) A carbon supply device for cultivating micro algae in large and its application method and use. CN200510126465.2/AU2006324198.
Su, Z. F., R. J. Kang, S. Y Shi, W. Cong, and Z. L. Cai (2008) An economical device for carbon supplement in large-scale microalgae production. Bioproc. Biosyst. Eng. 31: 641–645.
Vonshak, A. (1997) Outdoor mass production of Spirulina: The basic concept. pp. 79–99. In: Vonshak, A. (ed.) Spirulina platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology. Taylor and Francis, London, UK.
Belkin, S. and S. Boussiba (1991) Resistance of Spirulina platensis to ammonia at high pH values. Plant Cell Physiol. 32: 953–958.
Zarrouk, C. (1966) Contribution à l’étude d’une cyanophycée: influence de divers facteurs physiques et chimiques sur la croissance et la photosynthèse de Spirulina maxima. Ph.D. Thesi. University of Paris, Paris, France.
Cong, W., R. J. Kang, and Z. L. Cai (2004) A pH-based feedback method for carbon sources control in cultivation of microalgae. Patent of China CN200410009360.4.
Fresenius, W., K. E. Quentin, and W. Schneider (1988) Water analysis: A practical guide to physico-chemical, chemical and microbiological water examination and quality assurance. pp. 247–251. Springer Verlag, Berlin, Heidelberg, Germany.
Xue, S. H., Z. F. Su, and W. Cong (2010) Growth of Spirulina platensis enhanced under intermittent illumination. J. Biotechnol. 151: 271–277.
Lívansky, K. and J. Doucha (1997) Additional CO2 saturation of thin-layer outdoor microalgal cultures: CO2 mass transfer and absorption efficiency. Algological Studies 87: 145–154.
Ryu, H. J., K. K. Oh, and Y. S. Kim (2009) Optimization of the influential factors for the improvement of CO2 utilization efficiency and CO2 mass transfer rate. J. Ind. Eng. Chem. 15: 471–475.
Livansky, K. (1982) Effect of temperature and pH on absorption of carbon dioxide by a free level of mixed solutions of some buffers. Folia Microbiol. 27: 55–59.
Araujo, S. C. and V. M. T. Garcia (2005) Growth biochemical composition of the diatom Chaetoceros cf. wighamii brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 246: 405–412.
Olaizola, M., E. O. Duerr, and D. W. Freeman (1991) Effect of CO2 enhancement in an outdoor algal production system using Tetraselmis sp. J. Appl. Phycol. 3: 363–366.
Chang, E. H. and S. S. Yang (2003) Some characteristics of microalgae isolated in Taiwan for biofixation of carbon dioxide. Bot. Bull. Acad. Sin. 44: 43–52.
Rosa, A. P. C., L. F. Carvalho, L. Goldbeck, and J. A. V. Costa (2011) Carbon dioxide fixation by microalgae cultivated in open bioreactors. Energ. Convers. Manage. 52: 3071–3073.
Doucha, J. and K. Lívansky (2006) Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgae (Chlorella sp.) photobioreactors operated in a Middle and Southern European climate. J. Appl. Phycolo. 18: 811–826.
Soletto, D., L. Binaghi, L. Ferrari, A. Lodi, J. C. M. Carvalho, M. Zilli, and A. Converti (2008) Effects of carbon dioxide feeding rate and light intensity on the fed-batch pulse-feeding cultivation of Spirulina platensis in helical photobioreactor. Biochem. Eng. J. 39: 369–375.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bao, Y., Liu, M., Wu, X. et al. In situ carbon supplementation in large-scale cultivations of Spirulina platensis in open raceway pond. Biotechnol Bioproc E 17, 93–99 (2012). https://doi.org/10.1007/s12257-011-0319-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-011-0319-9