Abstract
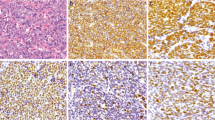
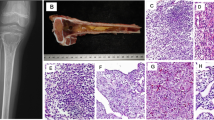
Over the years, a wide clinicopathological spectrum has been identified within Ewing family of tumors (EFTs). As these tumors are chemosensitive, their correct and timely identification is necessary. The aims of this study were (1) to present the diverse clinicopathological and molecular profile of EFTs in our settings, (2) to identify a pragmatic approach for diagnosing EFTs, especially for application of ancillary techniques, namely RT-PCR for specific transcripts (EWS-FLI1, EWS-ERG) and FISH for EWSR1 gene rearrangement, in certain cases and (3) to show the utility of tissue microarray in establishing a new FISH test. Fifty-eight EFTs were identified in 38 males and 20 females within an age-range of 1–65 years (median, 16), mostly in lower extremities (14) (24.1 %). Therapeutically, most patients underwent neoadjuvant chemotherapy with subsequent surgery. Histopathologically, diagnosis of EFTs was initially offered in 41/58 (70.6 %) tumors. On review, 59 % tumors showed diffuse pattern, while 41 % displayed rosettes. Immunohistochemically, tumor cells were mostly diffusely positive for CD99 (48/52) (92.3 %); FLI-1 (17/18) (94.4 %); variably for BCL2 (16/18) (88.8 %), synaptophysin (6/20) (35 %), S100-P (2/7) (28.5 %), CD56 (2/5) (40 %), NSE (2/5) (40 %), calponin (3/4) (75 %), EMA (5/24) (20.8 %) and CK (3/24) (12.5 %), the latter two mostly focally. Fifty five tumors were EWS-FLI1 positive, while a single tumor was EWS-ERG positive. Sensitivity for PCR was 61 %. EWSR1 rearrangement was detected by FISH in 12/13 Ewing sarcomas/PNETs. Sensitivity for EWSR1 test was 92.3 % and specificity was 100 %. Thirty-eight tumors, including 14 molecular confirmed EFTs and 21 other tumors were tested for EWSR1 rearrangement. Among 21 unrelated tumors, EWSR1 rearrangement was detected in few myoepithelial tumors, occasional desmoplastic small round cell tumor and an extraskeletal myxoid chondrosarcoma. Further, a tissue microarray with a separate set of 8 EFTs, confirmed at another laboratory was analysed for validation of EWSR1 rearrangement test. 23/28 (82.1 %) tissue cores of the tissue microarray, stained by FISH were interpretable, including EWSR1 rearrangement, detected in 20/28 tissue cores; not detected in 3 liver cores and uninterpretable in 5 (17.8 %) cores. Classical EFTs can be diagnosed with diffuse, membranous CD99 positivity, intranuclear FLI1 positivity and LCA negativity in malignant round cells. In unconventional cases, it is indispensable to reveal the concomitant fusion m-RNA by RT-PCR. In case of negative molecular results, it is necessary to prove EWSR1 rearrangement by FISH. These tests should be interpreted with clinicopathological correlation. Tissue microarrays for FISH are useful during validation of a new test, especially when sarcomas like EFTs show less genetic heterogeneity within tumor cells.






Similar content being viewed by others
References
Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, Ambros PF, Sheer D, Turc-Carel C, Triche TJ, Aurias A, Thomas G (1994) The Ewing family of tumors–a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med 331:294–299
Dome JS, Rodriguez-Galindo C, Spunt SL, Santana VM (2008) Pediatric solid tumors: Ewing’s sarcoma family tumors. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG (eds) Abeloff’s clinical oncology, 4th edn. Elsevier, Philadelphia, pp 2085–2091
Khoury JD (2005) Ewing sarcoma family of tumors. Adv Anat Pathol 12:212–220
Ushigome S, Machinami R, Sorensen PH (2002) Ewing sarcoma/Primitive neuroectodermal tumor. In: Fletcher CDM, Unni K, Mertens F (eds) Tumors of soft tissue and bone. Pathology and genetics. World Health Organization classification of tumors. IARC Press, Lyon, pp 298–300
Aurias A, Rimbaut C, Buffe D, Dubousset J, Mazabraud A (1983) Translocation of chromosome 22 in Ewing’s sarcoma. C R Seances Acad Sci III 296:1105–1107
Aurias A, Rimbaut C, Buffe D, Zucker JM, Mazabraud A (1984) Translocation involving chromosome 22 in Ewing’s sarcoma. A cytogenetic study of four fresh tumors. Cancer Genet Cytogenet 12:21–25
Zucman J, Melot T, Desmaze C, Ghysdael J, Plougastel B, Peter M, Zucker JM, Triche TJ, Sheer D, Turc-Carel C, Ambros P, Combaret V, Lenoir G, Aurias A, Thomas G, Delattre O (1993) Combinatorial generation of variable fusion proteins in the Ewing family of tumors. EMBO J 12:4481–4487
Urano F, Umezawa A, Yabe H, Hong W, Yoshida K, Fujinaga K, Hata J (1998) Molecular analysis of Ewing’s sarcoma: another fusion gene, EWS-E1AF, available for diagnosis. Jpn J Cancer Res 89:703–711
Shing DC, McMullan DJ, Roberts P, Smith K, Chin SF, Nicholson J, Tillman RM, Ramani P, Cullinane C, Coleman N (2003) FUS/ERG gene fusions in Ewing’s tumors. Cancer Res 63:4568–4576
Barr FG, Womer RB (2007) Molecular diagnosis of Ewing family tumors: too many fusions… ? J Mol Diagn 9:437–440
de Alava E, Kawai A, Healey JH, Fligman I, Meyers P, Huvos AG, Gerald WL, Jhanwar SC, Argani P, Antonescu CR, Pardo-Mindan FJ, Ginsberg J, Womer R, Lawlor ER, Wunder J, Andrulis I, Sorensen PHB, Barr FG, Ladanyi M (1998) EWS-FLI1 fusion transcript structure is an independent determinant of prognosis in Ewing’s sarcoma. J Clin Oncol 16:1248–1255
Hauben E, van den Broek LC, Van Marck E, Hogendoorn PC (2001) Adamantinoma-like Ewing’s sarcoma and Ewing’s-like adamantinoma. The t (11; 22), t (21; 22) status. J Pathol 195:218–221
Folpe AL, Goldblum JR, Rubin BP, Shehata BM, Liu W, Dei Tos AP, Weiss SW (2005) Morphologic and immunophenotypic diversity in Ewing family tumors: a study of 66 genetically confirmed cases. Am J Surg Pathol 29:1025–1033
Schuetz AN, Rubin BP, Goldblum JR, Shehata B, Weiss SW, Liu W, Wick MR, Folpe AL (2005) Intercellular junctions in Ewing sarcoma/primitive neuroectodermal tumor: additional evidence of epithelial differentiation. Mod Pathol 18:1403–1410
Llombart-Bosch A, Machado I, Navarro S, Bertoni F, Bacchini P, Alberghini M, Karzeladze A, Savelov N, Petrov S, Alvarado-Cabrero I, Mihaila D, Terrier P, Lopez-Guerrero JA, Picci P (2009) Histological heterogeneity of Ewing’s sarcoma/PNET: an immunohistochemical analysis of 415 genetically confirmed cases with clinical support. Virchows Arch 455:397–411
Ishii N, Hiraga H, Sawamura Y, Shinohe Y, Nagashima K (2001) Alternative EWS-FLI1 fusion gene and MIC2 expression in peripheral and central primitive neuroectodermal tumors. Neuropathology 21:40–44
Jimenez RE, Folpe AL, Lapham RL, Ro JY, O’Shea PA, Weiss SW, Amin MB (2002) Primary Ewing’s sarcoma/primitive neuroectodermal tumor of the Kidney. A clinicopathologic and immunohistochemical analysis of 11 cases. Am J Surg Pathol 26:320–327
Karpate A, Menon S, Basak R, Yuvaraja TB, Tongaonkar HB, Desai SB (2012) Ewing sarcoma/primitive neuroectodermal tumor of the kidney: clinicopathologic analysis of 34 cases. Ann Diagn Pathol 16:267–274
Gardner LJ, Ayala AG, Monforte HL, Dunphy CH (2004) Ewing sarcoma/peripheral primitive neuroectodermal tumor: adult abdominal tumors with an Ewing sarcoma gene rearrangement demonstrated by fluorescence in situ hybridization in paraffin sections. Appl Immunohistochem Mol Morphol 12:160–165
Rekhi B, Qureshi S, Basak R, Desai SB, Medhi S, Kurkure P, Menon S, Maheshwari A, Jambhekar NA (2010) Primary vaginal Ewing’s sarcoma or primitive neuroectodermal tumor in a 17-year-old woman: a case report. J Med Case Rep 4:88
Ostwal V, Rekhi B, Noronha V, Basak R, Desai SB, Maheshwari A, Prabhash K (2012) Primitive neuroectodermal tumor of ovary in a young lady, confirmed with molecular and cytogenetic results-a rare case report with a diagnostic and therapeutic challenge. Pathol Oncol Res 18:1104–1106.
Meier VS, Kühne T, Jundt G, Gudat F (1998) Molecular diagnosis of Ewing tumors: improved detection of EWS-FLI-1 and EWS-ERG chimeric transcripts and rapid determination of exon combinations. Diagn Mol Pathol 7:29–35
Yamaguchi U, Hasegawa T, Morimoto Y, Tateishi U, Endo M, Nakatani F, Kawai A, Chuman H, Beppu Y, Endo M, Kurotaki H, Furuta K (2005) A practical approach to the clinical diagnosis of Ewing’s sarcoma/primitive neuroectodermal tumor and other small round cell tumors sharing EWS rearrangement using new fluorescence in situ hybridisation probes for EWSR1 on formalin fixed, paraffin wax embedded tissue. J Clin Pathol 58:1051–1056
Bridge RS, Rajaram V, Dehner LP, Pfeifer JD, Perry A (2006) Molecular diagnosis of Ewing sarcoma/primitive neuroectodermal tumor in routinely processed tissue: a comparison of two FISH strategies and RT-PCR in malignant round cell tumors. Mod Pathol 19:1–8
Jambhekar NA, Bagwan IN, Ghule P, Shet TM, Chinoy RF, Agarwal S, Joshi R, Amare Kadam PS (2006) Comparative analysis of routine histology, immunohistochemistry, reverse transcriptase polymerase chain reaction, and fluorescence in situ hybridization in diagnosis of Ewing family of tumors. Arch Pathol Lab Med 130:1813–1818
Liu BY, Yang Y, Du J, Zhang Y, Wang H, Zheng J (2008) Application of the in situ hybridization with EWS dual-color break-apart fluorescence probe and anti-CD99 and anti-FLI-1 antibodies in the diagnosis of Ewing’s sarcoma/primitive neuroectodermal tumor. Beijing Da Xue Xue Bao 40:358–362
Gamberi G, Cocchi S, Benini S, Magagnoli G, Morandi L, Kreshak J, Gambarotti M, Picci P, Zanella L, Alberghini M (2011) Molecular diagnosis in Ewing family tumors: the Rizzoli experience–222 consecutive cases in four years. J Mol Diagn 13:313–324
Vural C, Uluoğlu O, Akyürek N, Oğuz A, Karadeniz C (2011) The evaluation of CD99 immunoreactivity and EWS/FLI1 translocation by fluorescence in situ hybridization in central PNETs and Ewing’s sarcoma family of tumors. Pathol Oncol Res 17:619–625
Qian X, Jin L, Shearer BM, Ketterling RP, Jalal SM, Lloyd RV (2005) Molecular diagnosis of Ewing’s sarcoma/primitive neuroectodermal tumor in formalin-fixed paraffin-embedded tissues by RT-PCR and fluorescence in situ hybridization. Diagn Mol Pathol 14:23–28
Bridge JA, Cushman-Vokoun AM (2011) Molecular diagnostics of soft tissue tumors. Arch Pathol Lab Med 135:588–601
Romeo S, Dei Tos AP (2010) Soft tissue tumors associated with EWSR1 translocation. Virchows Arch 456:219–234
Sugimura H, Mori H, Nagura K, Kiyose S, Tao H, Isozaki M, Igarashi H, Shinmura K, Hasegawa A, Kitayama Y, Tanioka F (2010) Fluorescence in situ hybridization analysis with a tissue microarray: ‘FISH and chips’ analysis of pathology archives. Pathol Int 60:543–550
Chen H, McClain D, Jhawar SC, Agaram NP, Hameed M (2012) Complex interphase fluorescent in situ hybridization patterns of EWSR1 gene in Ewing sarcoma using break apart probes. Abstract 28. USCAP
Kojima T, Asami S, Chin M, Yoshida Y, Mugishima H, Suzuki T (2002) Detection of chimeric genes in Ewing’s sarcoma and its clinical applications. Biol Pharm Bull 25:991–994
Parija T, Shirley S, Uma S, Rajalekshmy KR, Ayyappan S, Rajkumar T (2005) Type 1 (11; 22) (q24:q12) translocation is common in Ewing’s sarcoma/peripheral neuroectodermal tumor in south Indian patients. J Biosci 303:371–376
Chen N, Zhou Q (2005) Constructing tissue microarrays without prefabricating recipient blocks. A novel approach. Am J Clin Pathol 124:103–107
Machado I, Noguera R, Pellin A, Lopez-Guerrero JA, Piqueras M, Navarro S, Llombart-Bosch A (2009) Molecular diagnosis of ewing sarcoma family of tumors: a comparative analysis of 560 cases with FISH and RT-PCR. Diagn Mol Pathol 18:189–19938
Perlman EJ, Dickman PS, Askin FB, Grier HE, Miser JS, Link MP (1994) Ewing’s sarcoma–routine diagnostic utilization of MIC2 analysis: a Pediatric Oncology Group/Children’s Cancer Group Intergroup Study. Hum Pathol 25:304–307
Folpe AL, Hill CE, Parham DM, O’Shea PA, Weiss SW (2000) Immunohistochemical detection of FLI-1 protein expression: a study of 132 round cell tumors with emphasis on CD99-positive mimics of Ewing’s sarcoma/primitive neuroectodermal tumor. Am J Surg Pathol 24:1657–1662
Kavalar R, Pohar Marinsek Z, Jereb B, Cagran B, Golouh R (2009) Prognostic value of immunohistochemistry in the Ewing’s sarcoma family of tumors. Med Sci Monit 15:CR442–CR452
Mhawech-Fauceglia P, Herrmann F, Penetrante R, Beck A, Sait S, Block AM, Odunsi K, Fisher J, Balos L, Cheney RT (2006) Diagnostic utility of FLI-1 monoclonal antibody and dual-colour, break-apart probe fluorescence in situ (FISH) analysis in Ewing’s sarcoma/primitive neuroectodermal tumor (EWS/PNET). A comparative study with CD99 and FLI-1 polyclonal antibodies. Histopathology 49:569–575
Llombart-Bosch A, Navarro S (2001) Immunohistochemical detection of EWS and FLI-1 proteinss in Ewing sarcoma and primitive neuroectodermal tumors: comparative analysis with CD99 (MIC-2) expression. Appl Immunohistochem Mol Morphol 9:255–260
Downing JR, Head DR, Parham DM, Douglass EC, Hulshof MG, Link MP, Motroni TA, Grier HE, Curcio-Brint AM, Shapiro DN (1993) Detection of the (11;22)(q24;q12) translocation of Ewing’s sarcoma and peripheral neuroectodermal tumor by reverse transcription polymerase chain reaction. Am J Pathol 143:1294–1300
Sorensen PH, Liu XF, Delattre O, Rowland JM, Biggs CA, Thomas G, Triche TJ (1993) Reverse transcriptase PCR amplification of EWS/FLI-1 fusion transcripts as a diagnostic test for peripheral primitive neuroectodermal tumors of childhood. Diagn Mol Pathol 2:147–157
Dockhorn-Dworniczak B, Schäfer KL, Dantcheva R, Blasius S, Winkelmann W, Strehl S, Burdach S, van Valen F, Jürgens H, Böcker W (1994) Diagnostic value of the molecular genetic detection of the t(11;22) translocation in Ewing’s tumors. Virchows Arch 425:107–112
Guillou L (2008) Contribution of molecular biology and markers to the prognosis and management of patients with soft tissue sarcoma. Pathol Case Rev 13:69–77
Kumar R, Rekhi B, Shirazi N, Pais A, Amare P, Gawde D, Jambhekar N (2008) Spectrum of cytomorphological features, including literature review, of an extraskeletal myxoid chondrosarcoma with t(9;22)(q22;q12) (TEC/EWS) results in one case. Diagn Cytopathol 36:868–875
Bridge JA (2008) Contribution of cytogenetics to the management of poorly differentiated sarcomas. Ultrastruct Pathol 32:63–71
Diaz LK, Gupta R, Kidwai N, Sneige N, Wiley EL (2004) The use of TMA for interlaboratory validation of FISH testing for detection of HER2 gene amplification in breast cancer. J Histochem Cytochem 52:501–507
de Alava E, Antonescu CR, Panizo A, Leung D, Meyers PA, Huvos AG, Pardo-Mindán FJ, Healey JH, Ladanyi M (2000) Prognostic impact of P53 status in Ewing sarcoma. Cancer 89:783–792
Huang HY, Illei PB, Zhao Z, Mazumdar M, Huvos AG, Healey JH, Wexler LH, Gorlick R, Meyers P, Ladanyi M (2005) Ewing sarcomas with p53 mutation or p16/p14ARF homozygous deletion: a highly lethal subset associated with poor chemoresponse. J Clin Oncol 23:548–558
Balamuth NJ, Womer RB (2010) Ewing’s sarcoma. Lancet Oncol 11:184–1192
Pierron G, Tirode F, Lucchesi C, Reynaud S, Ballet S, Cohen-Gogo S, Perrin V, Coindre JM, Delattre O (2012) A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet 44:461–466
Italiano A, Sung YS, Zhang L, Singer S, Maki RG, Coindre JM, Antonescu CR (2012) High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosome Cancer 51:207–218
Ladanyi M, Bridge JA (2000) Contribution of molecular genetic data to the classification of sarcomas. Hum Pathol 31:532–538
Acknowledgments
This study (in part) was presented by B.R. at the Association for Molecular Pathologists (AMP) meeting on Genomic Medicine, 2012 at Long Beach, California USA, from 25th October to 27th October, 2012. The support of Immunohistochemistry laboratory of our Department is acknowledged. We thank Smita Mumbarkar, Jyoti Bodke and Rajni Mohite from molecular pathology laboratory, ACTREC, Khargar, Navi Mumbai. We acknowledge Ms Barbara Mankel, Institute of Pathology, University Hospital Tuebingen, Eberhard-Karls-University, Germany for technical support.
Financial disclosure
None.
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rekhi, B., Vogel, U., Basak, R. et al. Clinicopathological and Molecular Spectrum of Ewing Sarcomas/PNETs, Including Validation of EWSR1 Rearrangement by Conventional and Array FISH Technique in Certain Cases. Pathol. Oncol. Res. 20, 503–516 (2014). https://doi.org/10.1007/s12253-013-9721-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-013-9721-2