Abstract
Chronic myeloid leukemia (CML) is a cancer of blood cells driven by the BCR–ABL1 oncogenic protein tyrosine kinase, which is the product of a reciprocal chromosomal translocation known as the Philadelphia chromosome. Discovery of tyrosine kinase inhibitors targeting the BCR–ABL1 kinase revolutionized CML therapy, but these drugs are unable to eradicate the disease due to the presence of a drug-insensitive stem cell population that sustains continued growth of the malignant cells. Resistance to therapies also increases the risk of relapse and disease progression to a more advanced phase. This review discusses emerging issues in CML research, and describes recent progress in elucidating the mechanisms of CML stem cell maintenance and disease progression.
Similar content being viewed by others
Introduction
As we make progress toward effective therapies against cancer, several important issues remain, including therapy resistance, disease relapse following therapy cessation, and disease progression to advanced stages. Decades of studies on acute myeloid leukemia (AML) have provided sufficient evidence to conclude that leukemia is organized as a hierarchy of several distinct leukemic cell types with different degrees of self-renewal and differentiation potential [1]. There is a functionally, and often phenotypically, distinct population of tumor-initiating cells that exhibit the capacity to self-renew, the potential to generate all of the cell types present in the tumor, and the ability to proliferate actively and thereby drive sustained expansion of malignant cells [2, 3]. The tumor-initiating cells, or cancer stem cells, represent a therapy-resistant cell population and are primary drivers of cancer propagation and disease relapse after treatment and/or chemotherapy cessation [4, 5]. Recent studies have also supported the idea that several types of solid tumors, if not all, are a heterogeneous mixture of multiple cell types and are sustained by a sub-population with higher tumorigenic potential [6, 7]. It is thus reasonable and appealing to speculate that targeting a population that can maintain cancer growth may provide clues to effective treatment strategies and, ultimately, a cure to many hematologic malignancies and cancers. Indeed, in the mouse model of MOZ–TIF2-induced AML, forced cell death of the leukemia stem cell population leads to eradication of the disease in vivo [8]. In this Perspective, I will discuss emerging issues and the roles of novel players in stem cell maintenance and disease progression in cancer with a specific focus on chronic myeloid leukemia.
Chronic myeloid leukemia
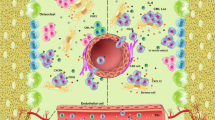
In the treatment of chronic myeloid leukemia (CML), chemotherapy resistance, disease relapse, and progression remain unsolved problems, and have been the focus of extensive research in recent years. CML is a clonal myeloproliferative disease characterized by the BCR–ABL1 fusion oncogene, which is the product of a reciprocal chromosomal translocation between chromosomes 9 and 22, known as the Philadelphia chromosome [9, 10]. BCR–ABL1 functions as a constitutively active oncogenic tyrosine kinase, and drives increased production of mature granulocytes (Fig. 1). Development of the tyrosine kinase inhibitor (TKI) imatinib mesylate revolutionized CML therapy, and over 70 % of patients with chronic phase CML (CP-CML) attain complete cytogenetic response and improved survival through the use of TKI [11, 12]. However, despite its effectiveness in controlling CP-CML, TKI is unable to eradicate the disease at the CML stem cell level, leading to long-term dependence on TKI, and increased risk of drug resistance and relapse following drug discontinuation, even after molecular remission [13, 14].
Development and disease progression in chronic myeloid leukemia. Chronic myeloid leukemia (CML) is initiated by the BCR–ABL1 translocation in hematopoietic stem cells, which leads to myeloid cell expansion while allowing differentiation (chronic phase CML). Secondary chromosomal translocations such as NUP98–HOXA9 or AML1–EVI1, or mutations in p53 or INK4A trigger disease progression to a more advanced phase (blast crisis CML), with progressive loss of the capacity to differentiate and increased expansion of immature blast cells. While tyrosine kinase inhibitors (TKIs) targeting BCR–ABL1 kinase activity can kill the oncogene-expressing progenitor cells, they are unable to eliminate the CML stem cells, leading to an increased risk of disease relapse after discontinuation of TKI therapy
Patients who show initial resistance to TKI and become resistant to primary TKI therapy are likely to relapse and progress to a more aggressive disease phase called blast crisis (Fig. 1) [15]. Compared with indolent CP-CML in which mature myeloid cells are produced, blast crisis CML (BC-CML) is characterized by the rapid proliferation of differentiation-arrested immature blast cells of either myeloid or lymphoid lineage [13, 14]. Interestingly, differentiation arrest and/or abnormal capacity of differentiation are shared features of hematologic malignancies and solid tumors with poor prognosis. Although the molecular mechanisms driving the transformation of CP to BC-CML are not fully understood, additional genetic mutations, defects in DNA damage repair and telomere maintenance are implicated in this process [13–17]. Unfortunately, due to its resistance to current therapies, BC-CML remains a major challenge in the treatment of CML.
Recently, it has become clear that while differentiated CML cells are dependent on BCR–ABL1 activity for their survival and therefore remain sensitive to kinase inhibition, CML stem cells do not rely on its activity and persist despite the effective inhibition of the BCR–ABL1 by TKIs [18–20]. These studies clearly highlight the need for targeting the BCR–ABL1-independent, alternative mechanisms that protect and sustain CML stem cells to develop curative therapies for this hematologic malignancy.
Mouse models of CML and insights for cancer stem cell research
Development of animal models that recapitulate many features of human CML have been invaluable to our efforts to gain a better understanding of the pathology and biology of CML. To date, three mouse models are well established and widely used in the field (Fig. 2). The most commonly used model utilizes retroviral transduction coupled with bone marrow transplantation (Fig. 2a) [21, 22]. In this model, hematopoietic stem cell (HSC)-enriched bone marrow cells from healthy or 5-fluorouracil-treated mice are transduced retrovirally with the BCR–ABL1 oncogene and the BCR–ABL1-expressing cells are transplanted into lethally irradiated mice. The recipient mice develop myeloproliferative disease within a few weeks that resembles many aspects of human CP-CML, including increased numbers of mature granulocytes in bone marrow and blood, splenomegaly, and anemia. In addition to CP-CML, human myeloid BC-CML can also be modeled in mice by combined retroviral expression of BCR–ABL1 and a second oncogene, such as NUP98–HOXA9 or AML1–EVI1 [23–26]. NUP98–HOXA9 is a product of the t(7;11) chromosomal translocation identified in humans, and is associated with blast crisis as well as de novo AML [26–28]. Recipient mice transplanted with cells expressing BCR–ABL1 plus NUP98–HOXA9 develop acute myeloproliferative disease that closely mimics human BC-CML. In contrast to the disease induced by the BCR–ABL1 oncogene alone, cells harboring the two oncogenic hits show immature morphology and immunophenotype and are highly tumorigenic upon serial transplantations [26].
Mouse models of CML. a Retroviral transduction-bone marrow (BM) transplantation model. To model CP-CML, hematopoietic stem cell (HSC)-enriched bone marrow cells from donor mice are transformed with BCR–ABL1 retrovirus and transplanted into lethally irradiated syngeneic recipient mice. For BC-CML-like acute leukemia, bone marrow cells are transduced with BCR–ABL1 and NUP98–HOXA9 retroviruses, and doubly infected cells are transplanted in sub-lethally irradiated recipient mice. b Inducible BCR–ABL1 transgenic mouse model. In a transgenic strain carrying the BCR–ABL1 transgene under the control of a Tet-responsive element and a second transgene encoding a Tet-regulated transactivator protein (tTA) driven by the SCL gene enhancer, BCR–ABL1 expression is induced by the removal of doxycycline, which leads to a CML-like disease in vivo. Alternatively, BCR–ABL1-expressing long-term HSC population is isolated by flow cytometry and transplanted into lethally irradiated recipients. c Human primary CML xenograft model. Primary CD34+ CML cells are isolated from patient specimens and transplanted into sublethally irradiated immunodeficient NOD–SCID or NSG mice
Another model of CML takes advantage of genetically engineered mice expressing the BCR–ABL1 transgene. Several distinct transgenic strains are available [29–32], and among those, a tetracycline(Tet)-regulated binary transgenic model is the most promising (Fig. 2b). This model carries the BCR–ABL1 transgene under the control of a Tet-responsive element as well as a second transgene encoding a Tet-regulated transactivator protein (tTA) driven by the SCL gene enhancer. The combination of these two transgenes results in Tet-regulated BCR–ABL1 expression in HSCs. BCR–ABL1 gene expression can be induced by the removal of doxycycline, a tetracycline analog, leading to a CML-like disease characterized by neutrophilia and splenomegaly. Using the retroviral/transplantation model, CML stem cells have been identified in the population with a Lin− Sca-1+ c-Kit+ (LSK) phenotype [33]. In a recent study using the binary BCR–ABL1-inducible model, Passegue and colleagues found that CML stem cells share the same cell surface phenotype as long-term HSCs (LSK Flk2− CD48− CD150+) [34]. Although the transplantation of BCR–ABL1-expressing cells with a short-term HSC phenotype (LSK Flk2− CD48− CD150−) can cause transient myeloid hyperplasia, these cells are unable to induce CML-like disease in vivo. In contrast, as few as 50 BCR–ABL1 + long-term HSCs lead to robust and long-lasting engraftment, and most of the engrafted mice eventually develop CML with an average latency of 4 months, demonstrating that CML stem cells reside exclusively in the long-term HSC compartment.
The most physiologically relevant mouse model currently available is a xenograft model of human primary CML cells (Fig. 2c). Flow-sorted primary CD34+ leukemia cells from patient bone marrow specimens are transplanted into immune-compromised recipient mice, such as NOD–SCID mice [35, 36]. Although the model remains technically challenging due to low efficiencies of long-term engraftment and/or biologic variations observed in primary leukemia specimens, development of the novel immunodeficient mouse strain NSG (NOD–SCID IL-2Rγ-chain-deficient) has generated an improved model with robust engraftment, providing a better way to analyze human CML stem cells in vivo and test the effectiveness of novel therapies against human CML [37].
Emerging players mediating stem cell maintenance and disease progression in CML
In the past decade, multiple signaling pathways have been identified as critical regulators of CML pathogenesis by the use of the aforementioned mouse models of CML. These include classic developmental pathways such as Wnt/beta-catenin and Hedgehog [38–40], as well as the multifunctional signaling scaffold protein β-arrestin [41], the promyelocytic leukemia protein (PML), transcriptional regulators of FoxO and HIF1α [42–44] and the arachidonate 5-lipoxygenase Alox5 [45] (well reviewed in [46–48]). Discussed below are the emerging players that sustain stem cells and drive cancer progression in CML.
Oncogenic RNA binding proteins
RNA binding proteins (RBPs) are key components in cellular RNA metabolism. RBPs regulate maturation, alternative splicing, nucleocytoplasmic transport, translation and stability of mRNAs and non-coding RNAs, and therefore altered RBP activity and expression can have profound effects on cellular events. Indeed, CML progression from chronic to blast crisis phase has been shown to accompany dysregulated expression of distinct RBPs, including heterogeneous nuclear ribonucleoproteins (hnRNPs), Lin28, and Musashi (Msi) (Fig. 3).
Dysregulation of RNA-binding proteins during CML progression from chronic to blast crisis phase. Expressions of RNA-binding proteins (RBPs) become elevated in BC-CML with concomitant decrease in the levels of their target RNAs. Inactivation of these RNA-binding proteins, or forced expression of the target RNA species can functionally rescue the arrested differentiation in BC-CML, suggesting that targeting these pathways may provide novel therapeutic strategies to block and reverse CML progression
Pioneering work on hnRNP E2 clearly showed that its gene expression is highly upregulated in human BC-CML compared with CP-CML, and that the hnRNP E2 protein binds to C/EBPα mRNA to repress its translation [49]. The transcription factor C/EBPα is essential for granulocyte differentiation, and functional inactivation of C/EBPα results in a block to myeloid differentiation. Thus, suppression of C/EBPα mRNA translation by upregulated hnRNP E2 protein is one of the mechanisms of differentiation arrest leading to the expansion of immature myeloid cells in BC-CML. A recent study has shown that miR-328, one of the microRNAs (miRNAs) downregulated during CML progression, competes with C/EBPα mRNA for binding to hnRNP E2 [50]. Forced expression of miR-328 increases C/EBPα protein levels in BC-CML cells and functionally impairs the clonogenic potential of human BC-CML cells, indicating that miR-328 functions as a decoy RNA to modulate hnRNP E2 activity.
RBPs also mediate regulation of miRNA biogenesis. The RNA binding protein Lin28 binds to let-7 miRNA precursors and inhibits their processing and maturation. While the let-7 family of miRNAs is abundant in differentiated cells, its expression is repressed in undifferentiated pluripotent ES cells and human cancers [51]. Consistent with its role in let-7 miRNA maturation, Lin28 is overexpressed in a broad range of human cancers [52]. Lin28 expression is more frequent in BC-CML specimens than in CP-CML, and the inhibition of Lin28 expression by RNA interference in K562 cells restores the levels of mature let-7 miRNA and impairs the proliferative and clonogenic capacities with concomitant induction of cellular differentiation, suggesting that targeting the Lin28-let-7 axis might delay or block the blast crisis transformation.
The Musashi gene (Msi) encodes an RBP originally identified as a developmental regulator of neural cell fate in the fruit fly. Later studies showed that there are two paralogous Msi genes in mammals (Msi1 and Msi2), and that the Msi1 gene expression marks tissue stem cells in brain, eye, and intestine [53]. Msi1 binds to and inhibits the translation of the mRNA for Numb, which specifies a differentiated cell fate in neural progenitors and HSCs [54, 55]. The functional importance of Msi1 in neural stem cells, however, remains elusive. Msi1 inactivation alone results in barely appreciable defects during embryonic neural development, which may be due to functional redundancies among the two paralogs [56]. The second gene, Msi2, is predominantly expressed in the most immature HSC compartment within the hematopoietic organ, and during CML disease progression, Msi2 expression levels are significantly elevated in the blast crisis phase [57]. Importantly, NUP98–HOXA9 can trigger Msi2 expression, which in turn represses Numb expression. Genetic loss of Msi2 results in increased Numb levels and, as a consequence, functionally impairs the development and propagation of BC-CML in vivo. Interestingly, Msi2 is not only highly upregulated during human CML progression, but is also a marker for poorer prognosis in human BC-CML and de novo AMLs [57, 58]. Collectively, these studies define the essential role of the RNA binding factor Msi2 as a molecular driver of cancer progression by regulating the balance between stem cell self-renewal and differentiation.
SIRT1 histone deacetylase
Histone deacetylases (HDACs) are among the promising candidates for targeted therapies in CML [36]. A recent study identified a non-canonical class III HDAC protein, SIRT1 as an exciting new target for eradicating CML stem cells [37]. SIRT1, the founding member of the Sirtuin family of proteins, is a mammalian homolog of the yeast protein Silent Information Regulator 2 (Sir2), and exerts a powerful influence on a variety of cellular processes, including DNA repair, cell cycle, metabolism and aging in diverse organisms [59]. Unlike canonical HDACs, SIRT1 requires the coenzyme nicotinamide adenine dinucleotide to deacetylate its targets, and it is not inhibited by pan-HDAC inhibitors [60]. In the CD34+ population from human CP- and BC-CML cells, SIRT1 is expressed at higher levels than in the CD34+ population in normal bone marrow [37]. SIRT1 downregulation by RNA interference or pharmacological inhibition of its activity by tenovin-6, a selective small molecule inhibitor of SIRT1, induces cell death and impairs the proliferation of the quiescent, TKI-insensitive CML stem cell population in culture. SIRT1 inhibition ex vivo reduces the growth of CML cells and compromises the long-term engraftment of human CD34+ CML cells upon transplantation into immunodeficient NSG mice. Spurred by the successful inhibition of CML stem cell growth in vitro, Li et al. tested whether tenovin-6 could impair the CML development in vivo by using the Tet-regulated BCR–ABL1 transgenic model. Although imatinib treatment alone reduced tumor burden, it failed to impact the CML stem cells. In contrast, combined treatment of imatinib and tenovin-6 effectively inhibited the maintenance of the BCR–ABL1 + long-term HSC population and significantly prolonged overall survival after discontinuation of the drug treatments. These results suggest that targeting SIRT1 could provide a novel therapeutic approach for the eradication of the CML stem cells.
Concluding remarks
Recent basic and translational research has contributed to our understanding of the biology of CML stem cells and has uncovered several potential candidates for targeted therapies in the myeloid leukemia. An accumulating body of evidence supports the idea that the properties of CML stem cells are shared by a wide array of cancer stem cells in other hematologic malignancies and solid tumors. Thus, the knowledge gained thus far from studies of CML is likely to have broader implications and extend to other cancers.
References
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7.
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8.
Kikushige Y, Shima T, Takayanagi S-I, Urata S, Miyamoto T, Iwasaki H, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7:708–17.
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–61.
Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–21.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60.
Aikawa Y, Katsumoto T, Zhang P, Shima H, Shino M, Terui K, et al.: PU.1-mediated upregulation of CSF1R is crucial for leukemia stem cell potential induced by MOZ–TIF2. Nat Med 2010;16:580–5.
Ren R. Mechanisms of BCR–ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–83.
Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808–17.
Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–52.
Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–53.
Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Investig. 2010;120:2254–64.
Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7:441–53.
Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–25.
Feinstein E, Cimino G, Gale RP, Alimena G, Berthier R, Kishi K, et al. p53 in chronic myelogenous leukemia in acute phase. Proc Natl Acad Sci USA. 1991;88:6293–7.
Mitani K, Ogawa S, Tanaka T, Miyoshi H, Kurokawa M, Mano H, et al. Generation of the AML1–EVI-1 fusion gene in the t(3;21)(q26;q22) causes blastic crisis in chronic myelocytic leukemia. EMBO J. 1994;13:504–10.
Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–25.
Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR–ABL activity. J Clin Investig. 2011;121:396–409.
Kumari A, Brendel C, Hochhaus A, Neubauer A, Burchert A. Low BCR–ABL expression levels in hematopoietic precursor cells enable persistence of chronic myeloid leukemia under imatinib. Blood. 2012;119:530–9.
Daley G, Van Etten R, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–30.
Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–92.
Cuenco GM, Ren R. Both AML1 and EVI1 oncogenic components are required for the cooperation of AML1/MDS1/EVI1 with BCR/ABL in the induction of acute myelogenous leukemia in mice. Oncogene. 2004;23:569–79.
Dash AB, Williams IR, Kutok JL, Tomasson MH, Anastasiadou E, Lindahl K, et al. A murine model of CML blast crisis induced by cooperation between BCR/ABL and NUP98/HOXA9. Proc Natl Acad Sci USA. 2002;99:7622–7.
Mayotte N, Roy D-C, Yao J, Kroon E, Sauvageau G. Oncogenic interaction between BCR–ABL and NUP98–HOXA9 demonstrated by the use of an in vitro purging culture system. Blood. 2002;100:4177–84.
Neering SJ, Bushnell T, Sozer S, Ashton J, Rossi RM, Wang PY, et al. Leukemia stem cells in a genetically defined murine model of blast-crisis CML. Blood. 2007;110:2578–85.
Ahuja HG, Popplewell L, Tcheurekdjian L, Slovak ML. NUP98 gene rearrangements and the clonal evolution of chronic myelogenous leukemia. Genes Chromosomes Cancer. 2001;30:410–5.
Yamamoto K, Nakamura Y, Saito K, Furusawa S. Expression of the NUP98/HOXA9 fusion transcript in the blast crisis of Philadelphia chromosome-positive chronic myelogenous leukaemia with t(7;11)(p15;p15). Br J Haematol. 2000;109:423–6.
Huettner CS, Zhang P, Van Etten RA, Tenen DG. Reversibility of acute B-cell leukaemia induced by BCR–ABL1. Nat Genet. 2000;24:57–60.
Huettner CSC, Koschmieder SS, Iwasaki HH, Iwasaki-Arai JJ, Radomska HSH, Akashi KK, et al. Inducible expression of BCR/ABL using human CD34 regulatory elements results in a megakaryocytic myeloproliferative syndrome. Blood. 2003;102:3363–70.
Bockamp E, Antunes C, Maringer M, Heck R, Presser K, Beilke S, et al. Tetracycline-controlled transgenic targeting from the SCL locus directs conditional expression to erythrocytes, megakaryocytes, granulocytes, and c-kit-expressing lineage-negative hematopoietic cells. Blood. 2006;108:1533–41.
Koschmieder S, Göttgens B, Zhang P, Iwasaki-Arai J, Akashi K, Kutok JL, et al. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR–ABL leukemogenesis. Blood. 2005;105:324–34.
Hu Y, Swerdlow S, Duffy TM, Weinmann R, Lee FY, Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci USA. 2006;103:16870–5.
Reynaud D, Pietras E, Barry-Holson K, Mir A, Binnewies M, Jeanne M, et al. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20:661–73.
Wang JC, Lapidot T, Cashman JD, Doedens M, Addy L, Sutherland DR, et al. High level engraftment of NOD/SCID mice by primitive normal and leukemic hematopoietic cells from patients with chronic myeloid leukemia in chronic phase. Blood. 1998;91:2406–14.
Zhang B, Strauss AC, Chu S, Li M, Ho Y, Shiang K-D, et al. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 2010;17:427–42.
Li L, Wang L, Li L, Wang Z, Ho Y, McDonald T, et al. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21:266–81.
Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of β-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–41.
Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–9.
Dierks C, Beigi R, Guo G-R, Zirlik K, Stegert MR, Manley P, et al. Expansion of Bcr–Abl-positive leukemic stem cells is dependent on hedgehog pathway activation. Cancer Cell. 2008;14:238–49.
Fereshteh M, Ito T, Kovacs JJ, Zhao C, Kwon HY, Tornini V, et al. β-Arrestin2 mediates the initiation and progression of myeloid leukemia. Proc Natl Acad Sci USA. 2012;109:12532–7.
Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–8.
Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, et al. TGF-β-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–80.
Zhang H, Li H, Xi HS, Li S. HIF1α is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119:2595–607.
Chen Y, Hu Y, Zhang H, Peng C, Li S. Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat Genet. 2009;41:783–92.
Helgason GV, Young GAR, Holyoake TL. Targeting chronic myeloid leukemia stem cells. Curr Hematol Malig Rep. 2010;5:81–7.
Deininger M. Stem cell persistence in chronic myeloid leukemia. Leukemia. 2012;1:S46–8.
Goldman JM, Gordon M, Bazeos A, Marin D. Biology of CML stem cells: the basis for clinical heterogeneity. Leukemia. 2012;1:S43–5.
Perrotti D, Cesi V, Trotta R, Guerzoni C, Santilli G, Campbell K, et al. BCR–ABL suppresses C/EBPα expression through inhibitory action of hnRNP E2. Nat Genet. 2002;30:48–58.
Eiring AM, Harb JG, Neviani P, Garton C, Oaks JJ, Spizzo R, et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010;140:652–65.
Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20:2202–7.
Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–8.
Okano H, Kawahara H, Toriya M, Nakao K, Shibata S, Imai T. Function of RNA-binding protein Musashi-1 in stem cells. Exp Cell Res. 2005;306:349–56.
Imai T, Tokunaga A, Yoshida T, Hashimoto M, Mikoshiba K, Weinmaster G, et al. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol. 2001;21:3888–900.
Wu M, Kwon HY, Rattis F, Blum J, Zhao C, Ashkenazi R, et al. Imaging hematopoietic precursor division in real time. Cell Stem Cell. 2007;1:541–54.
Sakakibara S-I, Nakamura Y, Yoshida T, Shibata S, Koike M, Takano H, et al. RNA-binding protein Musashi family: roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci USA. 2002;99:15194–9.
Ito T, Kwon HY, Zimdahl B, Congdon KL, Blum J, Lento WE, et al. Regulation of myeloid leukaemia by the cell-fate determinant Musashi. Nature. 2010;466:765–8.
Kharas MG, Lengner CJ, Al-Shahrour F, Bullinger L, Ball B, Zaidi S, et al. Musashi-2 regulates normal hematopoiesis and promotes aggressive myeloid leukemia. Nat Med. 2010;16:903–8.
Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–95.
Liu T, Liu PY, Marshall GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69:1702–5.
Acknowledgments
This work was supported in part by fellowships to T.I. from the Interdisciplinary Stem Cell Training Program by the California Institute of Regenerative Medicine, and from the Astellas Foundation for Research on Metabolic Disorders. I would like to thank Dr. Tannishtha Reya and the members of the Reya Laboratory for fruitful discussions, thoughtful insights and critical reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ito, T. Stem cell maintenance and disease progression in chronic myeloid leukemia. Int J Hematol 98, 641–647 (2013). https://doi.org/10.1007/s12185-013-1318-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-013-1318-8