Abstract
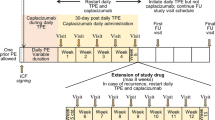
This phase II, multicenter, open-label, sequential-cohort, dose-escalation study was designed to evaluate the safety and efficacy of romiplostim, a novel peptibody that increases platelet production, in Japanese patients with chronic immune thrombocytopenic purpura (ITP). Sequential cohorts of four patients each received romiplostim (1, 3, or 6 μg/kg) subcutaneously on days 1 and 8 of the dose-escalation phase. Patients who achieved platelet responses (doubling of baseline platelet counts to ≥50 × 109/L) continued romiplostim weekly during the treatment-continuation phase. Romiplostim produced dose-dependent increases in mean and peak platelet counts. Five patients received romiplostim during the treatment-continuation phase, with platelet counts ≥50 × 109/L maintained in approximately half of the weekly assessments. Romiplostim was well tolerated. No severe, serious, or life-threatening adverse events were reported. No binding antibodies to romiplostim or thrombopoietin were detected. Romiplostim is safe and well tolerated in Japanese patients with chronic ITP and is effective in producing platelet count increases, consistent with the results from studies in non-Japanese patients. On the basis of these findings, a starting dose of 3 μg/kg was recommended for phase III evaluation of romiplostim in Japanese patients with chronic ITP.




Similar content being viewed by others
References
British Committee for Standards in Haematology General Hematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol. 2003;120:574–96. doi:10.1046/j.1365-2141.2003.04131.x.
Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi:10.1056/NEJMra010501.
George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88:3–40.
McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44:S3–11. doi:10.1053/j.seminhematol.2007.11.002.
Bromberg ME. Immune thrombocytopenic purpura: the changing therapeutic landscape. N Engl J Med. 2006;355:1643–5. doi:10.1056/NEJMp068169.
Cines DB, McMillan R. Management of adult idiopathic thrombocytopenic purpura. Annu Rev Med. 2005;56:425–42. doi:10.1146/annurev.med.56.082103.104644.
George JN, Kojouri K, Perdue JJ, Vesely SK. Management of patients with chronic, refractory idiopathic thrombocytopenic purpura. Semin Hematol. 2000;37:290–8. doi:10.1016/S0037-1963(00)90107-0.
Chang M, Nakagawa PA, Williams SA, Schwartz MR, Imfeld KL, Buzby JS, et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102:887–95. doi:10.1182/blood-2002-05-1475.
McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103:1364–9. doi:10.1182/blood-2003-08-2672.
Kosugi S, Kurata Y, Tomiyama Y, Tahara T, Kato T, Tadokoro S, et al. Circulating thrombopoietin level in chronic immune thrombocytopenic purpura. Br J Haematol. 1996;93:704–6. doi:10.1046/j.1365-2141.1996.d01-1702.x.
Mukai HY, Kojima H, Todokoro K, Tahara T, Kato T, Hasegawa Y, et al. Serum thrombopoietin (TPO) levels in patients with amegakaryocytic thrombocytopenia are much higher than those with immune thrombocytopenic purpura. Thromb Haemost. 1996;76:675–8.
Kuter DJ. New thrombopoietic growth factors. Blood. 2007;109:4607–16. doi:10.1182/blood-2006-10-019315.
Nomura S, Dan K, Hotta T, Fujimura K, Ikeda Y. Effects of pegylated recombinant human megakaryocyte growth and development factor in patients with idiopathic thrombocytopenic purpura. Blood. 2002;100:728–30. doi:10.1182/blood.V100.2.728.
Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241–8. doi:10.1182/blood.V98.12.3241.
Broudy VC, Lin NL. AMG531 stimulates megakaryopoiesis in vitro by binding to Mpl. Cytokine. 2004;25:52–60. doi:10.1016/j.cyto.2003.05.001.
Nichol JL. AMG 531: an investigational thrombopoiesis-stimulating peptibody. Pediatr Blood Cancer. 2006;47:723–5. doi:10.1002/pbc.20972.
Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355:1672–81. doi:10.1056/NEJMoa054626.
Newland A, Caulier MT, Kappers-Klunne M, Schipperus MR, Lefrere F, Zwaginga JJ, et al. An open-label, unit dose-finding study of AMG 531, a novel thrombopoiesis-stimulating peptibody, in patients with immune thrombocytopenic purpura. Br J Haematol. 2006;135:547–53. doi:10.1111/j.1365-2141.2006.06339.x.
Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther. 2004;76:628–38. doi:10.1016/j.clpt.2004.08.010.
Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi:10.1016/S0140-6736(08)60203-2.
Kumagai Y, Fujita T, Ozaki M, Sahashi K, Ohkura M, Ohtsu T, et al. Pharmacodynamics and pharmacokinetics of AMG 531, a thrombopoiesis-stimulating peptibody, in healthy japanese subjects: a randomized, placebo-controlled study. J Clin Pharmacol. 2007;47:1489–97. doi:10.1177/0091270007306563.
Nakagawa M. Idiopathic thrombocytopenic purpura: a practice guideline for diagnosis and treatment of intractable diseases. ed Rev. ed. Tokyo: Roppo shuppansha; 2001.
Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic itp. Blood. 2009;113:2161–71. doi:10.1182/blood-2008-04-150078.
Acknowledgments
This study was sponsored by Amgen KK (Japan). The authors would like to thank Yoshiaki Ogawa, Tokai University; and Kiyoshi Kitamura for providing for study support. M. Ohkura and Y. Sonehara, former employees of Amgen KK, provided biostatistical and study management support, respectively. Editorial support for the manuscript was provided by B. Weichman, PhD, and PAREXEL, and was funded by Amgen Inc.
Conflict of interest statement
D. P. Berger is an employee of Amgen Inc. T. Ohtsu is a former employee of Amgen KK. Y. Shirasugi, K. Ando, S. Hashino, T. Nagasawa, Y. Kurata, Y., Kishimoto, and K. Iwato received research funding from Amgen.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shirasugi, Y., Ando, K., Hashino, S. et al. A phase II, open-label, sequential-cohort, dose-escalation study of romiplostim in Japanese patients with chronic immune thrombocytopenic purpura. Int J Hematol 90, 157–165 (2009). https://doi.org/10.1007/s12185-009-0361-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-009-0361-y