Abstract
Background
Long life of memory T cell (Tm) determines its crucial role in the carcinogenesis and carcinogenic progression which usually take long time. The Tm compartment contains two populations, central memory T cells (Tcm) and effector memory T cells (Tem), based on their phenotypic markers, functional attributes, and migratory properties.
Methods
We investigated the subsets of the Tm in peripheral blood and tumor microenvironments in patients with gastric cancer by flow cytometry, and aimed to explore the correlation between the Tm and clinicopathologic features of gastric cancer.
Results
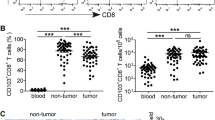
The percentages of CD4+/CD8+ Tm and CD4+/CD8+ Tcm in peripheral blood from gastric cancer patients were statistically lower, whereas the percentages of CD4+/CD8+ Tem were significantly higher than healthy controls. The proportion of CD4+/CD8+ Tcm increased after tumor resection, while the percentage of the CD4+/CD8+ Tem decreased significantly. Significant associations were detected between the peripheral CD4+/CD8+ Tm and clinical stage, as well as the CD8+ Tcm and clinical stage and nodal involvement. Tumor infiltrating CD8+ Tm expressed both central and effector memory phenotypes, whereas CD4+ Tm displayed predominantly an effector memory phenotype. Higher percentages of tumor infiltrating CD4+/CD8+ Tm were significantly associated with the early disease stage.
Conclusions
Tm and its subsets were good immune indicators for the disease stage of gastric cancer. The proportion of Tm subsets may reflect the immune suppressive and immune response to the tumor associated antigen.




Similar content being viewed by others
References
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12.
Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108(6):871–8.
Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4(3):225–34.
Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102(27):9571–6.
Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–24.
Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186(6):859–65.
Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007;179(12):8243–51.
Hwang ML, Lukens JR, Bullock TN. Cognate memory CD4+ T cells generated with dendritic cell priming influence the expansion, trafficking, and differentiation of secondary CD8+ T cells and enhance tumor control. J Immunol. 2007;179(9):5829–38.
Merkenschlager M, Terry L, Edwards R, Beverley PC. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988;18(11):1653–61.
Hotta K, Sho M, Fujimoto K, Shimada K, Yamato I, Anai S, et al. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011;105(8):1191–6.
Xiang R, Lode HN, Gillies SD, Reisfeld RA. T cell memory against colon carcinoma is long-lived in the absence of antigen. J Immunol. 1999;163(7):3676–83.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.
Belov L, Zhou J, Christopherson RI. Cell surface markers in colorectal cancer prognosis. Int J Mol Sci. 2010;12(1):78–113.
Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–102.
Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, et al. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138(4):1429–40.
Bindea G, Mlecnik B, Fridman WH, Galon J. The prognostic impact of anti-cancer immune response: a novel classification of cancer patients. Semin Immunopathol. 2011;33(4):335–40.
Hara M, Matsuzaki Y, Shimizu T, Tomita M, Ayabe T, Enomoto Y, et al. Preoperative peripheral naive/memory ratio and prognosis of nonsmall-cell lung cancer patients. Ann Thorac Cardiovasc Surg. 2007;13(6):384–90.
Kuss I, Schaefer C, Godfrey TE, Ferris RL, Harris JM, Gooding W, et al. Recent thymic emigrants and subsets of naive and memory T cells in the circulation of patients with head and neck cancer. Clin Immunol. 2005;116(1):27–36.
Sallusto F, Lanzavecchia A. Exploring pathways for memory T cell generation. J Clin Invest. 2001;108(6):805–6.
Barbieri C, Fujisawa MM, Yasuda CL, Metze IL, Oliveira EC, Santos LM, et al. Effect of surgical treatment on the cellular immune response of gastric cancer patients. Braz J Med Biol Res. 2003;36(3):339–45.
Tanel A, Fonseca SG, Yassine-Diab B, Bordi R, Zeidan J, Shi Y, et al. Cellular and molecular mechanisms of memory T-cell survival. Expert Rev Vaccin. 2009;8(3):299–312.
Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–62.
Rauser S, Langer R, Tschernitz S, Gais P, Jutting U, Feith M, et al. High number of CD45RO+ tumor infiltrating lymphocytes is an independent prognostic factor in non-metastasized (stage I–IIA) esophageal adenocarcinoma. BMC Cancer. 2010;10:608.
Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–66.
Broussard EK, Disis ML. TNM staging in colorectal cancer: T is for T cell and M is for memory. J Clin Oncol. 2011;29(6):601–3.
Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222(4):350–66.
Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99(10):1704–11.
Acknowledgments
This work was supported partially by the Tianjin Natural Science Funds (no. 13JCYBJC24200 and 13JCQNJC12700).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Zhang and F. Li contributed equally.
Rights and permissions
About this article
Cite this article
Zhang, R., Li, F., Li, H. et al. The clinical significance of memory T cells and its subsets in gastric cancer. Clin Transl Oncol 16, 257–265 (2014). https://doi.org/10.1007/s12094-013-1066-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-013-1066-5