Abstract
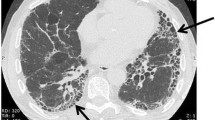
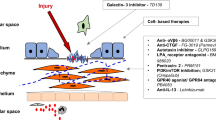
Interstitial lung disease is a rare side effect of temsirolimus treatment in renal cancer patients. Pulmonary fibrosis is characterised by the accumulation of extracellular matrix collagen, fibroblast proliferation and migration, and loss of alveolar gas exchange units. Previous studies of pulmonary fibrosis have mainly focused on the fibro-proliferative process in the lungs. However, the molecular mechanism by which sirolimus promotes lung fibrosis remains elusive. Here, we propose an overall cascade hypothesis of interstitial lung diseases that represents a common, partly underlying synergism among them as well as the lung pathogenesis side effects of mammalian target of rapamycin inhibitors.
Similar content being viewed by others
References
Abraham RT (2004) PI3-kinase related kinases: “big” players in stress-induced signalling pathways. DNA Repair (Amst) 3:883–887
Chen J, Zheng XF, Brown EJ, Schreiber SL (1995) Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA 92:4947–4951
Jacinto E, Loewith R, Schmidt A et al (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6:1122–1128
Sarbassov DD, Ali SM, Kim DH et al (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14:1296–1302
Chan S (2004) Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer 91:1420–1424
Atkins MB, Hidalgo M, Stadler WM et al (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22:909–918
Duran I, Siu LL, Oza AM et al (2006) Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer 42:1875–1880
Morelon E, Stern M, Kreis H (2000) Interstitial pneumonitis associated with sirolimus therapy in renal-transplant recipients. N Engl J Med 343:225–226
Morelon E, Manzer-Bruneel MF, Peraldi MN, Kreis H (2001) Sirolimus; a new promising immunosuppressive drug. Towards a rationale for its use in renal transplantation. Nephrol Dial Transplant 16:18–20
Pham PT, Pham PC, Danovitch GM et al (2004) Sirolimus-associated pulmonary toxicity. Transplantation 77:1215–1220
Singer SJ, Tiernan R, Sullivan EJ (2000) Interstitial pneumonitis associated with sirolimus therapy in renal-transplant recipients. N Engl J Med 343:1815–1816
Mahalati K (2000) Bronchiolitis obliterans and organizing pneumonia in renal transplant recipients. Transplantation 69:1581
Vignot S, Faivre S, Aguirre D, Raymond E (2005) mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol 16:525–537
Chan S, Scheulen ME, Johnston S et al (2005) Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 23:5314–5322
Galanis E, Buckner JC, Maurer MJ et al; North Central Cancer Treatment Group (2005) Phase II trial of emsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol 23:5294–5304
Witzig TE, Geyer SM, Ghobrial I et al (2005) Phase II trial of single-agent Temsirolimus (CCI-779) for relapse mantle cell lymphoma. J Clin Oncol 23:5347–5356
Israel-Biet D, Labrune S, Huchon GJ (1991) Drug-induced lung disease: review, 1990. Eur Respir J 4:465–478
Kilburn KH (1980) Pulmonary disease induced by drugs. In: Fishman AP (ed.) Pulmonary disease and disorders. McGraw-Hill, New York, pp 707–724
Rosenow EC, Myers JL, Swensen SJ, Pisani RJ (1992) Drug-induced pulmonary disease: an update. Chest 102:239–250
Vahid B, Marik PE (2008) Pulmonary complications of novel antineoplastic agents for solid tumors. Chest 133:528–538
Weis RB, Muggia FM (1980) Cytotoxic druginduced pulmonary disease: update. Am J Med 68:259–266
Whimster WF, de Poitiers W (1982) The lung. In: Riddell RH (ed.) Pathology of drug-induced and toxic diseases. Churchill Livingstone, New York, pp 167–200
Katzebstein ALA (1985) Pathogenesis of “fibrosis” in interstitial pneumonia: an electron microscopic study. Hum Pathol 16:1015–1024
Crystal RG, Bitterman PB, Rennard SI et al (1984) Interstitial lung disease of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract. N Engl J Med 310:154–166
Rennard S, Bitterman P, Crystal R (1983) Response of the lower respiratory tract to injury. Mechanisms of repair of the parenchymal cells of the alveolar wall. Chest 84:735–739
Liebow A (1975) Definition and classification of interstitial pneumonia in human pathology. Prog Respir Res 8:1–33
Spencer H (1975) Pathogenesis of interstitial fibrosis of the lung. Prog Respir Res 8:34–44
Hussain SP, Hofseth LJ, Haris CC (2003) Radical causes of cancer. Nat Rev Cancer 3:276–285
Ohshima H, Tatemichi M, Sawa T (2003) Chemical basis of inflammation-induced carcinogenesis. Arch Biochem Biophys 417:3–11
Chen LF, Williams SA, Mu Y et al (2005) NFkappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol 25:7966–7975
Kiernan R (2003) Post-activation turn-off NFkappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem 278:2758–2766
Barnes PJ, Karin M (1997) Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–1071
Ghosh S, Karin M (2002) Missing pieces in the NF-kB puzzle. Cell 109:S81–96
Hoffmann A, Levchenko A, Scott ML, Baltimore D (2002). The IkappaB-NF-kappaB signalling module: temporal control and selective gene activation. Science 298:1241–1245
Hoffmann A, Leung TH, Baltimore D (2003) Genetic analysis of NF-kappaB/rel transcription factors defines functional specificities. EMBO J 22:5530–5539
Jacobs MN, Harrison SD (1998) Structure of an I-kBa/NF-kB complex. Cell 95:749–758
Chen LW, Egan L, Li ZW et al (2003) The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med 9:575–581
Li Q, Verma IM (2002) NF-kB regulation in the immune system. Nat Rev Immunol 2:725–734
Maeda S, Chang L, Li ZW et al (2003) IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNF alpha. Immunity 19:725–737
Moore KJ, Rosen ED, Fitzgerald ML et al (2001) The role of PPAR-gamma in macrophage differentiation and cholesterol uptake. Nat Med 7:41–47
Petersen KF, Dufour S, Befroy D et al (2004) Impaired mitochondrial activity in the insulinresistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671
Castranova VD, Porter L, Millecchia JY et al (2002) Effect of inhaled crystalline silica in rat model: time course of pulmonary reactions. Mol Cell Biochem 234–235:177–184
Shukla A, Ramos-Nino M, Mossman B (2003) Cell signalling and transcription factor activation by asbestos in lung injury and disease. Int J Biochem Cell Biol 35:1198–1209
Arslan SO, Zerin M, Vural H, Coskun A (2002) The effect of melatonin on bleomycin-induced pulmonary fibrosis in rats. J Pineal Res 32:21–25
Chen J, Stubbe J (2004) Bleomycins: a new methods will allow reinvestigation of old issues. Curr Opin Cell Biol 8:175–181
Hubbard AK, Timblin CR, Shukla A et al (2002) Activation of NF-kB-dependent gene expression by silica in lungs of luciferase reporter mice. Am J Physiol Lung Cell Mol Physiol 282:L968–975
Benjamin RC, Gill DM (1980) Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strands breaks. J Biol Chem 255:10502–10508
Cosi C, Marien M (1999) Implication of poly(ADP-ribose)polymerase (PARP) in neurodegeneration and brain energy metabolism. Decreases in mouse brain NAD+ and ATP caused by MPTP are prevented by the PARP inhibitor benzamide. Ann NY Acad Sci 890:227–239
Virag L (2005) Poly(ADP-ribosyl)ation in asthma and other lung diseases. Pharmacol Res 52:83–92
Fattman CL, Chang LY, Termin TA et al (2003) Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med 35:763–771
Griffith OW, Stuehr DJ (1995) Nitric oxide synthase: properties and catalytic mechanism. Annu Rev Physiol 57:707–736
Halliwell B (1997) What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett 411:157–160
Adamsson IY, Bowden DH (1974) The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 77:185–197
Fasske E, Morgenroth K (1983) Experimental bleomycin lung in mice. A contribution to the pathogenesis of pulmonary fibrosis. Lung 161:133–146
Chakrabarti S, Makrigiorgos GM, O’Brien K et al (1996) Measurement of hydroxyl radicals catalyzed in the immediate vicinity of DNA by metal-bleomycin complexes. Free Radic Biol Med 20:777–783
Conley NS, Yarbro JW, Ferrari HA, Zeidler RB (1986) Bleomycin increases superoxide anion generation by pig peripheral alveolar macrophages. Mol Pharmacol 30:48–52
Tarnell EW, Oliver BL, Johnsson GM et al (1992) Superoxide anion production by rat neutrophils at various stages of belomycin-induced lung injury. Lung 170:41–50
Moncada S, Palmer RM, Higgs EA (1989) Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol 38:1709–1715
Forstermann U, Gath I, Schwarz P et al (1995) Isoforms of nitric oxide synthase. Properties, cellular distribution and expressional control. Biochem Pharmacol 50:1321–1332
Cuzzocrea S, Zingarelli B, Hake P et al (1998) Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic Biol Med 24:450–459
Wei XD, Charles IG, Smith A et al (1995) Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408–411
Gansauge S, Gansauge F, Nussler AK et al (1997) Exogenous, but not endogenous, nitric oxide increases proliferation rates in senescent human fibroblasts. FEBS Lett 410:160–164
Romanska HM, Polak JM, Colemena RA et al (2002) iNOS gene upregulation is associated with the early proliferative response of human lung fibroblasts to cytokine stimulation. J Pathol 197:372–379
Saleh D, Barnes PJ, Giaid A (1997) Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 155:1763–1769
Ask K, Maretin GE, Kolb M, Gauldie J (2006) Targeting genes for treatment in idiopathic pulmonary fibrosis: challenges and opportunities, promises and pitfalls. Proc Am Thorac Soc 3:389–393
Sporn MB, Roberts AB, Wakefield LM, de Crombrugghe B (1987). Some recent advances in the chemistry and biology of transforming growth factor-β. J Cell Biol 105:1039–1045
Fine A, Goldstein RH (1987) The effect of transforming growth factor-β on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem 262:3897–3902
Moses HL, Branum EL, Proper JA, Robinson RA (1981) Transforming growth factor production by chemically transformed cells. Cancer Res 41:2842–2848
Roberts AB, Lamb LC, Newton DL et al (1980) Transforming growth factors: isolation of polypeptides from virally and chemical transformed cells by acid/ethanol extraction. Proc Natl Acad Sci USA 77:3494–3498
Gotzman J, Mikula M, Eger A et al (2004) Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res 566:9–20
Tosh D, Slack JM (2002) How cells changes their phenotype. Nat Rev Mol Cell Biol 3:187–194
Roberts AB, Sporn MB (1993) Physiological actions and clinical applications of transforming growth factor-β (TFG-β). Growth Factors 8:1–9
Janda E, Lehmann K, Killisch I et al (2002) Ras and TGF(beta) cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signalling pathways. J Cell Biol 156:299–313
Peinado H, Quintanilla M, Cano A (2003) Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transition. J Biol Chem 278:21113–21123
Hoyt DG, Lazo JS (1988) Alterations in pulmonary mRNA encoding procollagens, fibronectin and transforming growth factor-β precede bleomycin-induced pulmonary fibrosis in mice. J Pharmacol Exp Ther 246:765–771
Barral-Neto M, Barral A, Brownel CE et al (1992) Transforming growth factor β in leishmanial infection: a parasite escape mechanism. Science 257:545–548
Zhang K, Rekhter MD, Gordon D, Phan SH (1994) Localization of myofibroblasts in the lung, and their role in collagen gene expression in a model of pulmonary fibrosis. Am J Pathol 145:114–125
Li MO, Wan YY, Sanjabi S et al (2006) Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24:99–146
Rubtsov YP, Rudensky AY (2007) TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol 7:443–453
Khalil N, Corne S, Whitman C, Yacyshyn H (1996) Plasmin regulates the activation of cell-associated latent TGF-β secreted by rat alveolar macrophages after in vivo bleomycin injury. Am J Resp Cell Mol Biol 15:252–259
Broekelmann TJ, Limper AH, Colby TV, McDonald JA (1991) Transforming growth factor β1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA 88:6642–664
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by an unrestricted educational grant from Pfizer
Rights and permissions
About this article
Cite this article
Aparicio, G., Calvo, M.B., Medina, V. et al. Comprehensive lung injury pathology induced by mTOR inhibitors. Clin Transl Oncol 11, 499–510 (2009). https://doi.org/10.1007/s12094-009-0394-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-009-0394-y