Abstract
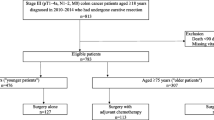
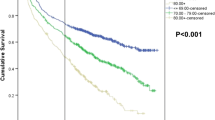
Randomized trials conducted in the 1980s have established the effectiveness of 5-fluorouracil-based adjuvant chemotherapy in treating stage-III colon cancer. However, the initiation of adjuvant chemotherapy is just the first step for survival improvement. Little is known about the actual completion rate of such a therapy in the community. The objectives of this study were to measure the initiation and completion rate of adjuvant chemotherapy and to identify the associated factors. We studied 12,265 patients aged 65+ diagnosed with stage-III colon cancer between 1991 and 2005 who were identified from the Surveillance, Epidemiology, and End Results-Medicare linked database. Chemotherapy initiation was defined as at least one claim indicating the use of chemotherapy. The first and last claims were used to measure the length of chemotherapy. A complete course of chemotherapy was defined as 8–13 months for 1991–1995 cohort and 5–7 months for 1996–2005 cohort according to clinical guideline. Of the 12,265 patients, 64.4% received adjuvant chemotherapy within 3 months after tumor resection. Among those who had chemotherapy initiated, 62.2% (or 38.0% of 12,265 patients) received a complete course of chemotherapy. Patient’s age at diagnosis, marital status, and comorbidity score were the significant predictors for chemotherapy initiation. These variables remained significant in predicting chemotherapy completion after adjusting for year of diagnosis and other factors. In conclusion, initiation and completion of chemotherapy was largely influenced by patient’s age, marital status and comorbidity. Further investigation is needed to explore the cause of these differences in adherence to standard treatment that is essential for better quality of cancer care.


Similar content being viewed by others
References
Horner MJ, Ries LAG, Krapcho M, et al. SEER cancer statistics review. Bethesda, MD, National Cancer Institute; 1975–2006.
Laurie JA, Moertel CG, Fleming TR, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol. 1989;7:1447–56.
Erlichman C, Fine S, Wong A, et al. A randomized trial of fluorouracil and folinic acid in patients with metastatic colorectal carcinoma. J Clin Oncol. 1988;6:469–75.
Poon MA, O’Connell MJ, Moertel CG, et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7:1407–18.
The Nordic Gastrointestinal Tumor Adjuvant Therapy Group. Superiority of sequential methotrexate, fluorouracil, and leucovorin to fluorouracil alone in advanced symptomatic colorectal carcinoma: a randomized trial. J Clin Oncol. 1989;7:1437–46.
Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–8.
NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. Jama. 1990; 264:1444–50.
Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995; 345:939–44.
Engstrom PF, Benson AB 3rd, Cohen A, et al. NCCN colorectal cancer practice guidelines. The national comprehensive cancer network. Oncology (Williston Park). 1996;10:140–75.
Neugut AI, Matasar M, Wang X, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006;24:2368–75.
O’Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16:295–300.
Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005;23:8671–8.
International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. J Clin Oncol. 1999;17:1356–63.
Moertel CG, Fleming TR, Macdonald JS, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes’ B2 colon cancer. J Clin Oncol. 1995;13:2936–43.
Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93:850–7.
Steele GD, Jessup JM. Colorectal cancer. In: Steele GD, Jessup JM, Winchester DP, et al., editors. National cancer data base: annual review of patients care 1995. Atlanta, GA: American Cancer Society; 1995.
Ragland KE, Selvin S, Merrill DW. Black-white differences in stage-specific cancer survival: analysis of seven selected sites. Am J Epidemiol. 1991;133:672–82.
Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst. 2001;93:501–15.
Du X, Goodwin JS. Patterns of use of chemotherapy for breast cancer in older women: findings from Medicare claims data. J Clin Oncol. 2001;19:1455–61.
Cooper GS, Yuan Z, Stange KC, et al. Agreement of Medicare claims and tumor registry data for assessment of cancer-related treatment. Med Care. 2000;38:411–21.
Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:IV-55–61.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Limited-Use Data (1973–2006), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2009, based on the November 2007 submission.
Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–48.
Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–9.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–9. discussion 1081-90.
SEER-Medicare: calculation of comorbidity weights, National Cancer Institute (Accessed 4 Dec 2009).
Potosky AL, Harlan LC, Kaplan RS, et al. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002;20:1192–202.
Hamel MB, Teno JM, Goldman L, et al. Patient age and decisions to withhold life-sustaining treatments from seriously ill, hospitalized adults. SUPPORT investigators. Study to understand prognoses and preferences for outcomes and risks of treatment. Ann Intern Med. 1999;130:116–25.
Weeks JC. Preferences of older cancer patients: can you judge a book by its cover? J Natl Cancer Inst. 1994;86:1743–4.
Muss HB, Cohen HJ, Lichtman SM. Clinical research in the older cancer patient. Hematol Oncol Clin North Am. 2000;14:283–91.
Sudano JJ, Baker DW. Explaining US racial/ethnic disparities in health declines and mortality in late middle age: the roles of socioeconomic status, health behaviors, and health insurance. Soc Sci Med. 2006;62:909–22.
Du XL, Meyer TE, Franzini L. Meta-analysis of racial disparities in survival in association with socioeconomic status among men and women with colon cancer. Cancer. 2007;109:2161–70.
SEER-medicare data training (Nov 16–17, 2009). National Cancer Institute; 2009.
Acknowledgments
We acknowledge the efforts of the National Cancer Institute, Center for Medicare and Medicaid Services, Information Management Services, Inc.; and the SEER tumor registries in the creation of this database. The analyses, interpretation, and reporting are the sole responsibilities of the authors. This study was supported by a grant from the Agency for Healthcare Research and Quality (RO1-HS016743).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, CY., Delclos, G.L., Chan, W. et al. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol 28, 1062–1074 (2011). https://doi.org/10.1007/s12032-010-9644-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9644-7