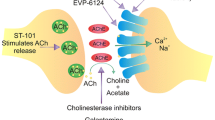
Abstract
Alzheimer’s disease is a progressive neurological disorder characterized by amyloid plaques and neurofibrillary tangles along with memory and cognitive deficits associated with a loss of basal forebrain cholinergic neurons. Efforts to treat Alzheimer’s disease have focused on compounds that elevate cholinergic activity such as cholinesterase inhibitors and direct acting muscarinic and nicotinic agonists. Low efficacy and poor selectivity of available compounds have limited the clinical utility of muscarinic agonists. Recent studies suggesting a role for muscarinic agonists in regulating the production of Aβ raise the possibility that selective M1 agonists could be useful in treating not only the symptoms, but also the underlying cause(s) of Alzheimer’s disease. Thus, renewed efforts have focused on the development of compounds with improved selectivity for M1 receptors and lower toxicity. 5-(3-ethyl-1,2,4-oxadiazol-5-yl)-1,4,5,6-tetrahydropyrimidine (CDD-0102) is a potent M1 agonist with a low side effect profile that enhances memory function in animal models of Alzheimer’s disease. The available preclinical data suggest that CDD-0102 may be useful in the treatment of Alzheimer’s disease.
Similar content being viewed by others
References
Bartus R. T., Dean R. L. D., Beer B., and Lippa A. S. (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217(4558), 408–414.
Bodick N. C., Offen W. W., Levey A. I., Cutler N. R., Gauthier S. G., et al. (1997) Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 54(4), 465–473.
Brann M. R., Buckley N. J., Jones S. V., and Bonner T. I. (1987) Expression of a cloned muscarinic receptor in A9 L cells. Mol. Pharmacol. 32(4), 450–455.
Buxbaum J. D., Ruefli A. A., Parker C. A., Cypess A. M., and Greengard P. (1994) Calcium regulates processing of the Alzheimer amyloid protein precursor in a protein kinase C-independent manner. Proc. Natl. Acad. Sci. USA 91(10), 4489–4493.
Bymaster F. P., Carter P. A., Peters S. C., Zhang W., Ward J. S., Mitch C. H., et al. (1998) Xanomeline compared to other muscarinic agents on stimulation of phosphoinositide hydrolysis in vivo and other cholinomimetic effects. Brain Res. 795(1–2), 179–190.
Caulfield M. P., Higgins G. A., and Straughan D. W. (1983) Central administration of the muscarinic receptor subtype-selective antagonist pirenzepine selectively impairs passive avoidance learning in the mouse. J. Pharm. Pharmacol. 35(2), 131–132.
Dornan W. A., McCampbell A. R., Tinkler G. P., Hickman L. J., Bannon A. W., Decker M. W., and Gunther K. L. (1997) Comparison of site specific injections into the basal forebrain on water maze and radial arm maze performance in the male rat after immunolesioning with 192 IgG saporin. Behav. Brain. Res. 86(2), 181–189.
Dunbar P. G., Durant G. J., Fang Z., Abuh Y. F., El-Assadi A. A., Ngur D. O., et al. (1993) Design, synthesis, and neurochemical evaluation of 5-(3-alkyl-1,2,4- oxadiazol-5-yl)-1,4,5,6-tetrahydropyrimidines as M1 muscarinic receptor agonists. J. Med. Chem. 36(7), 842–847.
Dunbar P. G., Durant G. J., Rho T., Ojo B., Huzl J. J. 3rd, Smith D. A., et al. (1994) Design, synthesis, and neurochemical evaluation of 2-amino-5- (alkoxycarbonyl)-3,4,5,6-tetrahydropyridines and 2-amino-5-(alkoxycarbonyl)-1,4,5,6-tetrahydropyrimidines as M1 muscarinic receptor agonists. J. Med. Chem. 37(17), 2774–2782.
Fonnum F. (1975) A rapid radiochemical method for the determination of choline acetyltransferase. J. Neurochem. 24(2), 407–409.
Hagan J. J., Jansen J. H., and Broekkamp C. L. (1987) Blockade of spatial learning by the M1 muscarinic antagonist pirenzepine. Psychopharmacology 93(4), 470–476.
Hock C., Maddalena A., Heuser I., Naber D., Oertel W., von der Kammer H., et al. (2000) Treatment with the selective muscarinic agonist talsaclidine decreases cerebrospinal fluid levels of Aβ in patients with Alzheimer’s disease. Soc. Neurosci. New Orleans, LA (abstract).
Hollander E., Mohs R. C., and Davis K. L. (1986) Cholinergic approaches to the treatment of Alzheimer’s disease. Br. Med. Bull. 42(1), 97–100.
Hoss W., Woodruff J. M., Ellerbrock B. R., Periyasamy S., Ghodsi-Hovsepian S., Stibbe J., et al. (1990) Biochemical and behavioral responses of pilocarpine at muscarinic receptor subtypes in the CNS. Comparison with receptor binding and low- energy conformations. Brain Res. 533(2), 232–238.
Huang X. P., Williams F. E., Peseckis S. M., and Messer W. S., Jr. (1998) Pharmacological characterization of human m1 muscarinic acetylcholine receptors with double mutations at the junction of TM VI and the third extracellular domain. J. Pharmacol. Exp. Ther. 286(3), 1129–1139.
Iga Y., Arisawa H., Ogane N., Saito Y., Tomizuka T., Nakagawa-Yagi Y., et al. (1998) (+/−)-cis-2-methylspiro[1,3-oxathiolane-5,3′-quinuclidine] hydrochloride, hemihydrate (SNI-2011, cevimeline hydrochloride) induces saliva and tear secretions in rats and mice: the role of muscarinic acetylcholine receptors. Jpn. J. Pharmacol. 78(3), 373–380.
Levey A. I. (1996) Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc. Natl. Acad. Sci. USA 93(24), 13541–13546.
Li X. (1998) Determination of CDD-0102-J in rat serum by high performance liquid chromatography and ultrafiltration. Department of Pharmacology, The University of Toledo, Toledo, OH, pp. 1–52.
Messer W. S. Jr., Abuh Y. F., Ryan K., Shepherd M. A., Schroeder M., Abunada S., Sehgal R., and El-Assadi A. A. (1997) Tetrahydropyrimidine derivatives display functional selectivity for M1 muscarinic receptors in brain. Drug Dev. Res. 40, 171–184.
Messer W. S. Jr., Abuh Y. F., Liu Y., Periyasamy S., Ngur D. O., Edgar M. A., El-Assadi A. A., et al. (1997) Synthesis and biological characterization of 1,4,5,6-tetrahydropyrimidine and 2-amino-3,4,5,6-tetrahydropyridine derivatives as selective m1 agonists. J. Med. Chem. 40(8), 1230–1246.
Messer W. S. Jr., Bohnett M., and Stibbe J. (1990) Evidence for a preferential involvement of M1 muscarinic receptors in representational memory. Neurosci. Lett. 116(1–2), 184–189.
Messer W. S. Jr., Rajeswaran W. G., Cao Y., Zhang H. J., El-Assadi A. A., Dockery C., et al. (2000) Design and development of selective muscarinic agonists for the treatment of Alzheimer’s disease: characterization of tetrahydropyrimidine derivatives and development of new approaches for improved affinity and selectivity for M1 receptors. Pharm. Acta Helv. 74(2–3), 135–140.
Messer W. S. Jr., Stibbe J. R., and Bohnett M. (1991) Involvement of the septohippocampal cholinergic system in representational memory. Brain Res. 564(1), 66–72.
Moos W. H., Davis R. E., Schwarz R. D., and Gamzu E. R. (1988) Cognition activators. Med. Res. Rev. 8(3), 353–391.
Murga C., Fukuhara S., and Gutkind J. S. (2000) A novel role for phosphatidylinositol 3-kinase beta in signaling from G protein-coupled receptors to Akt. J. Biol. Chem. 275(16), 12069–12073.
Murga C., Laguinge L., Wetzker R., Cuadrado A., and Gutkind J. S. (1998) Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinase-gamma. J. Biol. Chem. 273(30), 19080–19085.
Nitsch R. M., Deng M., Tennis M., Schoenfeld D., and Growdon J. H. (2000) The selective muscarinic M1 agonist AF102B decreases levels of total A[beta] in cerebrospinal fluid of patients with Alzheimer’s disease. Ann. Neurol. 48, 913–918.
Nitsch R. M., Slack B. E., Wurtman R. J., and Growdon J. H. (1992) Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 258(5080), 304–307.
Ojo B., Dunbar P. G., Durant G. J., Nagy P. I., Huzl J. J. 3rd., Periyasamy S., et al. (1996) Synthesis and biochemical activity of novel amidine derivatives as m1 muscarinic receptor agonists. Bioorg. Med. Chem. 4(10), 1605–1615.
Perry E. K., Tomlinson B. E., Blessed G., Bergmann K., Gibson P. H., and Perry R. H. (1978) Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br. Med. J. 2(6150), 1457–1459.
Schwarz R. D., Callahan M. J., Coughenour L. L., Dickerson M. R., Kinsora J. J., Lipinski W. J., et al. (1999) Milameline (CI-979/RU35926): a muscarinic receptor agonist with cognition-activating properties: biochemical chemical and in vivo characterization. J. Pharmacol. Exp. Ther. 291(2), 812–822.
Wall S. J., Yasuda R. P., Hory F., Flagg S., Martin B. M., Ginns E. I., and Wolfe, B. B. (1991) Production of antisera selective for m1 muscarinic receptors using fusion proteins: distribution of m1 receptors in rat brain. Mol. Pharmacol. 39(5), 643–649.
Walsh T. J., Herzog C. D., Gandhi C., Stackman R. W., and Wiley R. G. (1996) Injection of IgG 192-saporin into the medial septum produces cholinergic hyypofunction and dose-dependent working memory deficits. Brain Res. 726(1–2), 69–79.
Watson J. M., Hunter A. J., Brown A. M., and Middlemiss D. N. (1999) In vitro characterisation of the muscarinic receptor partial agonist, sabcomeline, in rat cortical and heart membranes. Eur. J. Pharmacol. 370(1), 69–77.
Weihl C. C., Ghadge G. D., Kennedy S. G., Hay N., Miller R. J., and Roos R. P. (1999) Mutant presenilin-1 induces apoptosis and downregulates Akt/PKB. J. Neurosci. 19(13), 5360–5369.
Wess J., Bonner T. I., and Brann M. R. (1990) Chimeric m2/m3 muscarinic receptors: role of carboxyl terminal receptor domains in selectivity of ligand binding and coupling to phosphoinositide hydrolysis. Mol. Pharmacol. 38(6), 872–877.
Whitehouse P. J., Price D. L., Struble R. G., Clark A. W., Coyle J. T., and Delon M. R. (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215(4537), 1237–1239.
Wolf B. A., Wertkin A. M., Jolly Y. C., Yasuda R. P., Wolfe B. B., Konrad R. J., et al. (1995) Muscarinic regulation of Alzheimer’s disease amyloid precursor protein secretion and amyloid beta-protein production in human neuronal NT2N cells. J. Biol. Chem. 270(9), 4916–4922.
Wood M. D., Murkitt K. L., Ho M., Watson J. M., Brown F., Hunter A. J., and Middlemiss D. N. (1999) Functional comparison of muscarinic partial agonists at muscarinic receptor subtypes hM1, hM2, hM3, hM4 and hM5 using microphysiometry. Br. J. Pharmacol. 126(7), 1620–1624.
Wrenn C. C., Lappi D. A., and Wiley R. G. (1999) Threshold relationship between lesion extent of the cholinergic basal forebrain in the rat and working memory impairment in the radial maze. Brain Res. 847(2), 284–298.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Messer, W.S. The utility of muscarinic agonists in the treatment of alzheimer’s disease. J Mol Neurosci 19, 187–193 (2002). https://doi.org/10.1007/s12031-002-0031-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s12031-002-0031-5