Abstract
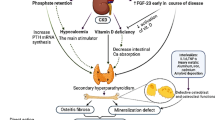
Bone lesions, collectively known as renal osteodystrophy (ROD), are a common complication of chronic kidney disease (CKD). Besides osteitis fibrosa and mixed lesions, other bone and mineral disorders such as adynamic bone disease, osteomalacia, osteoporosis, dialysis-related amyloidosis, and calcific uremic arteriolopathy are increasingly recognized in patients with CKD. Although bone lesions usually begin early in the course of CKD and are progressive, symptoms and signs such as bone pain and fractures may not occur until the patient is already on maintenance dialysis. More importantly, these disorders are associated with increased risk of cardiovascular disease and mortality in patients with CKD. The term ROD does not reflect the full spectrum of bone pathology or clinical manifestations of bone and mineral disorders in patients with CKD. Accordingly, the National Kidney Foundation and, more recently the Kidney Disease: Improving Global Outcomes, now consider ROD to represent only one measure of the skeletal component of the broader syndrome of chronic kidney disease-mineral and bone disorders in which abnormalities in bone and mineral metabolism or extraskeletal calcification are observed. In this review, we will discuss, in detail, the epidemiology, pathogenesis, histopathology, clinical manifestation, diagnosis, and treatment of these disorders.










Similar content being viewed by others
References
Andress DL. Adynamic bone in patients with chronic kidney disease. Kidney Int. 2008;73:1345–54.
Sherrard DJ, Hercz G, Pei Y, et al. The spectrum of bone disease in end-stage renal failure–an evolving disorder. Kidney Int. 1993;43:436–42.
Hercz G, Pei Y, Greenwood C, et al. Aplastic osteodystrophy without aluminum: the role of “suppressed” parathyroid function. Kidney Int. 1993;44:860–6.
Kurz P, Monier-Faugere MC, Bognar B, et al. Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int. 1994;46:855–61.
London GM, Marty C, Marchais SJ, et al. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–51.
KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009:S1–130.
Lehmann G, Ott U, Kaemmerer D, et al. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease Stages 3–5. Clin Nephrol. 2008;70:296–305.
Moore C, Yee J, Malluche H, et al. Relationship between bone histology and markers of bone and mineral metabolism in African-American hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1484–93.
Brandenburg VM, Floege J. Adynamic bone disease–bone and beyond. NDT Plus. 2008;1:135–47.
K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42:S1–201.
Changsirikulchai S, Domrongkitchaiporn S, Sirikulchayanonta V, et al. Renal osteodystrophy in Ramathibodi Hospital: histomorphometry and clinical correlation. J Med Assoc Thai. 2000;83:1223–32.
Cunningham J, Sprague SM, Cannata-Andia J, et al. Osteoporosis in chronic kidney disease. Am J Kidney Dis. 2004;43:566–71.
Martin KJ, Olgaard K, Coburn JW, et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004;43:558–65.
Rocha LA, Higa A, Barreto FC, et al. Variant of adynamic bone disease in hemodialysis patients: fact or fiction? Am J Kidney Dis. 2006;48:430–6.
Spasovski GB, Bervoets AR, Behets GJ, et al. Spectrum of renal bone disease in end-stage renal failure patients not yet on dialysis. Nephrol Dial Transplant. 2003;18:1159–66.
D’Haese PC, Spasovski GB, Sikole A, et al. A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int. 2003;63:S73–8.
Ferreira A, Frazao JM, Monier-Faugere MC, et al. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol. 2008;19:405–12.
Moe SM, Drueke TB. A bridge to improving healthcare outcomes and quality of life. Am J Kidney Dis. 2004;43:552–7.
Cannata-Andia JB. Hypokinetic azotemic osteodystrophy. Kidney Int. 1998;54:1000–16.
Saidak Z, Brazier M, Kamel S, et al. Agonists and allosteric modulators of the calcium-sensing receptor and their therapeutic applications. Mol Pharmacol [Review]. 2009;76:1131–44.
Barreto FC, Barreto DV, Moyses RM, et al. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73:771–7.
Monier-Faugere MC, Geng Z, Mawad H, et al. Improved assessment of bone turnover by the PTH-(1–84)/large C-PTH fragments ratio in ESRD patients. Kidney Int. 2001;60:1460–8.
Qunibi W, Kalantar-Zadeh K. Target levels for serum phosphorus and parathyroid hormone. Semin Dialysis. 2011;24:29–33.
Slatopolsky E. The interaction of parathyroid hormone and aluminum in renal osteodystrophy. Kidney Int. 1987;31:842–54.
Iwasaki-Ishizuka Y, Yamato H, Nii-Kono T, et al. Downregulation of parathyroid hormone receptor gene expression and osteoblastic dysfunction associated with skeletal resistance to parathyroid hormone in a rat model of renal failure with low turnover bone. Nephrol Dial Transplant. 2005;20:1904–11.
Massry SG, Stein R, Garty J, et al. Skeletal resistance to the calcemic action of parathyroid hormone in uremia: role of 1, 25 (OH)2 D3. Kidney Int. 1976;9:467–74.
Picton ML, Moore PR, Mawer EB, et al. Down-regulation of human osteoblast PTH/PTHrP receptor mRNA in end-stage renal failure. Kidney Int. 2000;58:1440–9.
Heaf J. Adynamic bone disease and malnutrition-inflammation-cachexia syndrome. Kidney Int 2007;71:1326; author reply 7.
Kalantar-Zadeh K, Shah A, Duong U, et al. Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int. 2010;78:S10–21.
Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney International [Congresses Research Support, Non-U.S. Gov’t]. 2008;73:391–8.
Sanchez-Gonzalez MC, Lopez-Barea F, Bajo MA, et al. Serum albumin levels, an additional factor implicated in hyperparathyroidism outcome in peritoneal dialysis: a prospective study with paired bone biopsies. Adv Perit Dial. 2006;22:198–202.
Carlstedt E, Ridefelt P, Lind L, et al. Interleukin-6 induced suppression of bovine parathyroid hormone secretion. Biosci Rep. 1999;19:35–42.
Amerling R, Harbord NB, Pullman J, et al. Bisphosphonate use in chronic kidney disease: association with adynamic bone disease in a bone histology series. Blood Purif. 2010;29:293–9.
Goodman WG, Ramirez JA, Belin TR, et al. Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int. 1994;46:1160–6.
Hamdy NA, Kanis JA, Beneton MN, et al. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ. 1995;310:358–63.
Balint E, Marshall CF, Sprague SM. Effect of the vitamin D analogues paricalcitol and calcitriol on bone mineral in vitro. Am J Kidney Dis. 2000;36:789–96.
Malluche HH, Mawad H, Monier-Faugere MC. Effects of treatment of renal osteodystrophy on bone histology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S157–63.
Spasovski GB. Bone biopsy as a diagnostic tool in the assessment of renal osteodystrophy. Int J Artif Organs. 2004;27:918–23.
Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945–53.
Herberth J, Monier-Faugere MC, Mawad HW, et al. The five most commonly used intact parathyroid hormone assays are useful for screening but not for diagnosing bone turnover abnormalities in CKD-5 patients. Clin Nephrol. 2009;72:5–14.
Gupta A, Kallenbach LR, Zasuwa G, et al. Race is a major determinant of secondary hyperparathyroidism in uremic patients. J Am Soc Nephrol. 2000;11:330–4.
Herberth J, Branscum AJ, Mawad H, et al. Intact PTH combined with the PTH ratio for diagnosis of bone turnover in dialysis patients: a diagnostic test study. Am J Kidney Dis. 2010;55:897–906.
Coen G, Ballanti P, Bonucci E, et al. Bone markers in the diagnosis of low turnover osteodystrophy in haemodialysis patients. Nephrol Dial Transplant. 1998;13:2294–302.
Lindberg JS, Moe SM. Osteoporosis in end-state renal disease. Semin Nephrol. 1999;19:115–22.
Danese MD, Kim J, Doan QV, et al. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47:149–56.
Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36:1115–21.
Yajima A, Ogawa Y, Ikehara A, et al. Development of low-turnover bone diseases after parathyroidectomy and autotransplantation. Int J Urol. 2001;8:S76–9.
Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58:396–9.
Rudser KD, de Boer IH, Dooley A, et al. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol. 2007;18:2401–7.
Haris A, Sherrard DJ, Hercz G. Reversal of adynamic bone disease by lowering of dialysate calcium. Kidney Int. 2006;70:931–7.
London GM, Marchais SJ, Guerin AP, et al. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol. 2008;19:1827–35.
Asci G, Ok E, Savas R, et al. The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol Dial Transplant. 2011;26:1010–5.
Barreto DV, Barreto FC, Carvalho AB, et al. Coronary calcification in hemodialysis patients: the contribution of traditional and uremia-related risk factors. Kidney Int. 2005;67:1576–82.
Mawad HW, Sawaya BP, Sarin R, et al. Calcific uremic arteriolopathy in association with low turnover uremic bone disease. Clin Nephrol. 1999;52:160–6.
Jean G, Lataillade D, Genet L, et al. Association between very low PTH Levels and poor survival rates in haemodialysis patients: results from the French ARNOS cohort. Nephron Clin Pract. 2010;118:c211–6.
Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–80.
Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–8.
Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–18.
Nakai S, Akiba T, Kazama J, et al. Effects of serum calcium, phosphorous, and intact parathyroid hormone levels on survival in chronic hemodialysis patients in Japan. Ther Apher Dial. 2008;12:49–54.
Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int. 2008;73:1296–302.
Dukkipati R, Kovesdy CP, Colman S, et al. Association of relatively low serum parathyroid hormone with malnutrition-inflammation complex and survival in maintenance hemodialysis patients. J Ren Nutr. 2010;20:243–54.
Tangri N, Wagner M, Griffith JL, et al. Effect of bone mineral guideline target achievement on mortality in incident dialysis patients: an analysis of the United Kingdom Renal Registry. Am J Kidney Dis. 2011;57:415–21.
Kuizon BD, Goodman WG, Juppner H, et al. Diminished linear growth during intermittent calcitriol therapy in children undergoing CCPD. Kidney Int. 1998;53:205–11.
van Driel M, Koedam M, Buurman CJ, et al. Evidence that both 1alpha, 25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J Cell Biochem. 2006;99:922–35.
Baldock PA, Thomas GP, Hodge JM, et al. Vitamin D action and regulation of bone remodeling: suppression of osteoclastogenesis by the mature osteoblast. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research [Comparative StudyResearch Support, Non-U.S. Gov’t]. 2006;21:1618–26.
Fretz JA, Zella LA, Kim S, et al. 1, 25-Dihydroxyvitamin D3 induces expression of the Wnt signaling co-regulator LRP5 via regulatory elements located significantly downstream of the gene’s transcriptional start site. J Steroid Biochem Mol Biol [Research Support, N.I.H., Extramural]. 2007;103:440–5.
Panda DK, Miao D, Bolivar I, et al. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. The Journal of biological chemistry [Research Support, Non-U.S. Gov’t]. 2004;279:16754–66.
Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–56.
Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–25.
Mathew S, Lund RJ, Chaudhary LR, et al. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008;19:1509–19.
Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–52.
Raggi P, James G, Burke SK, et al. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res. 2005;20:764–72.
Jamal SA, Hodsman AB. Reducing the risk of re-fracture in the dialysis population: is it time to consider therapy with PTH analogues? Semin Dial. 2011;24:12–5.
Fujimori A, Yorifuji M, Sakai M, et al. Low-calcium dialysate improves mineral metabolism in hemodialysis patients. Clin Nephrol. 2007;67:20–4.
Lezaic V, Pejanovic S, Kostic S, et al. Effects of lowering dialysate calcium concentration on mineral metabolism and parathyroid hormone secretion: a multicentric study. Ther Apher Dial. 2007;11:121–30.
Spasovski G, Gelev S, Masin-Spasovska J, et al. Improvement of bone and mineral parameters related to adynamic bone disease by diminishing dialysate calcium. Bone. 2007;41:698–703.
Cejka D, Kodras K, Bader T, et al. Treatment of Hemodialysis-Associated Adynamic Bone Disease with Teriparatide (PTH(1–34)): A Pilot Study. Kidney Blood Press Res. 2010;33:221–6.
Silver J, Bushinsky D. Harnessing the parathyroids to create stronger bones. Curr Opin Nephrol Hypertens. 2004;13:471–6.
Goodman WG, Hladik GA, Turner SA, et al. The Calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol. 2002;13:1017–24.
Ishii H, Wada M, Furuya Y, et al. Daily intermittent decreases in serum levels of parathyroid hormone have an anabolic-like action on the bones of uremic rats with low-turnover bone and osteomalacia. Bone. 2000;26:175–82.
Gowen M, Stroup GB, Dodds RA, et al. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest. 2000;105:1595–604.
Jaffe JA, Liftman C, Glickman JD. Frequency of elevated serum aluminum levels in adult dialysis patients. Am J Kidney Dis. 2005;46:316–9.
Araujo SM, Ambrosoni P, Lobao RR, et al. The renal osteodystrophy pattern in Brazil and Uruguay: an overview. Kidney Int. 2003;63:S54–6.
Jorgetti V, Lopez BD, Caorsi H, et al. Different patterns of renal osteodystrophy in Iberoamerica. Am J Med Sci. 2000;320:76–80.
Hruska KA, Teitelbaum SL. Renal osteodystrophy. N Engl J Med. 1995;333:166–74.
Felsenfeld AJ, Gutman RA, Llach F, et al. Osteomalacia in chronic renal failure: a syndrome previously reported only with maintenance dialysis. Am J Nephrol. 1982;2:147–54.
Nebeker HG, Coburn JW. Aluminum and renal osteodystrophy. Annu Rev Med. 1986;37:79–95.
Andress DL, Kopp JB, Maloney NA, et al. Early deposition of aluminum in bone in diabetic patients on hemodialysis. N Engl J Med. 1987;316:292–6.
Pei Y, Hercz G, Greenwood C, et al. Renal osteodystrophy in diabetic patients. Kidney Int. 1993;44:159–64.
Vincenti F, Arnaud SB, Recker R, et al. Parathyroid and bone response of the diabetic patient to uremia. Kidney Int. 1984;25:677–82.
Ott SM. Histomorphometric measurements of bone turnover, mineralization, and volume. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S151–6.
Kausz AT, Antonsen JE, Hercz G, et al. Screening plasma aluminum levels in relation to aluminum bone disease among asymptomatic dialysis patients. Am J Kidney Dis. 1999;34:688–93.
D’Haese PC, Couttenye MM, Goodman WG, et al. Use of the low-dose desferrioxamine test to diagnose and differentiate between patients with aluminium-related bone disease, increased risk for aluminium toxicity, or aluminium overload. Nephrol Dial Transplant. 1995;10:1874–84.
Netter P, Kessler M, Burnel D, et al. Aluminum in the joint tissues of chronic renal failure patients treated with regular hemodialysis and aluminum compounds. J Rheumatol. 1984;11:66–70.
Baker LR, Muir JW, Sharman VL, et al. Controlled trial of calcitriol in hemodialysis patients. Clin Nephrol. 1986;26:185–91.
Felsenfeld AJ, Rodriguez M, Coleman M, et al. Desferrioxamine therapy in hemodialysis patients with aluminum-associated bone disease. Kidney Int. 1989;35:1371–8.
Malluche HH, Smith AJ, Abreo K, et al. The use of deferoxamine in the management of aluminium accumulation in bone in patients with renal failure. N Engl J Med. 1984;311:140–4.
McCarthy JT, Milliner DS, Johnson WJ. Clinical experience with desferrioxamine in dialysis patients with aluminium toxicity. Q J Med. 1990;74:257–76.
Nickolas TL, Leonard MB, Shane E. Chronic kidney disease and bone fracture: a growing concern. Kidney Int. 2008;74:721–31.
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–95.
Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int. 2006;70:1358–66.
Taal MW, Roe S, Masud T, et al. Total hip bone mass predicts survival in chronic hemodialysis patients. Kidney Int. 2003;63:1116–20.
Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–50.
Huang GS, Chu TS, Lou MF, et al. Factors associated with low bone mass in the hemodialysis patients–a cross-sectional correlation study. BMC Musculoskelet Disord. 2009;10:60.
Lindergard B, Johnell O, Nilsson BE, et al. Studies of bone morphology, bone densitometry and laboratory data in patients on maintenance hemodialysis treatment. Nephron. 1985;39:122–9.
Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994;843:1–129.
Yamaguchi T, Kanno E, Tsubota J, et al. Retrospective study on the usefulness of radius and lumbar bone density in the separation of hemodialysis patients with fractures from those without fractures. Bone. 1996;19:549–55.
Jamal SA, Hayden JA, Beyene J. Low bone mineral density and fractures in long-term hemodialysis patients: a meta-analysis. Am J Kidney Dis. 2007;49:674–81.
Desmet C, Beguin C, Swine C, et al. Falls in hemodialysis patients: prospective study of incidence, risk factors, and complications. Am J Kidney Dis. 2005;45:148–53.
Park JC, Kovesdy CP, Duong U, et al. Association of serum alkaline phosphatase and bone mineral density in maintenance hemodialysis patients. Hemodial Int. 2010;14:182–92.
Miller PD. The kidney and bisphosphonates. Bone 2011.
Hodsman AB. Fragility fractures in dialysis and transplant patients. Is it osteoporosis, and how should it be treated? Perit Dial Int. 2001;21(Suppl 3):S247–55.
Miller PD, Roux C, Boonen S, et al. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20:2105–15.
Jamal SA, Bauer DC, Ensrud KE, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res. 2007;22:503–8.
Lu KC, Yeung LK, Lin SH, et al. Acute effect of pamidronate on PTH secretion in postmenopausal hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 2003;42:1221–7.
Miller PD. Fragility fractures in chronic kidney disease: an opinion-based approach. Cleve Clin J Med. 2009;76:715–23.
Westenfeld R, Ketteler M, Brandenburg VM. Anti-RANKL therapy–implications for the bone-vascular-axis in CKD? Denosumab in post-menopausal women with low bone mineral density. Nephrol Dial Transplant. 2006;21:2075–7.
Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res. 2011;26:1829–35.
Danesh F, Ho LT. Dialysis-related amyloidosis: history and clinical manifestations. Semin Dial. 2001;14:80–5.
Drueke TB, Massy ZA. Beta2-microglobulin. Semin Dial. 2009;22:378–80.
Yamamoto S, Kazama JJ, Narita I, et al. Recent progress in understanding dialysis-related amyloidosis. Bone. 2009;45(Suppl 1):S39–42.
Warren DJ, Otieno LS. Carpal tunnel syndrome in patients on intermittent haemodialysis. Postgrad Med J. 1975;51:450–2.
Kenzora JE. Dialysis carpal tunnel syndrome. Orthopedics. 1978;1:195–203.
Gejyo F, Yamada T, Odani S, et al. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem Biophys Res Commun. 1985;129:701–6.
Schwalbe S, Holzhauer M, Schaeffer J, et al. Beta 2-microglobulin associated amyloidosis: a vanishing complication of long-term hemodialysis? Kidney Int. 1997;52:1077–83.
Jadoul M, Garbar C, Noel H, et al. Histological prevalence of beta 2-microglobulin amyloidosis in hemodialysis: a prospective post-mortem study. Kidney Int. 1997;51:1928–32.
Otsubo S, Kimata N, Okutsu I, et al. Characteristics of dialysis-related amyloidosis in patients on haemodialysis therapy for more than 30 years. Nephrol Dial Transplant. 2009;24:1593–8.
Winchester JF, Salsberg JA, Levin NW. Beta-2 microglobulin in ESRD: an in-depth review. Adv Ren Replace Ther. 2003;10:279–309.
Copley JB, Lindberg JS. Nontransplant therapy for dialysis-related amyloidosis. Semin Dial. 2001;14:94–8.
Gejyo F, Homma N, Suzuki Y, et al. Serum levels of beta 2-microglobulin as a new form of amyloid protein in patients undergoing long-term hemodialysis. N Engl J Med. 1986;314:585–6.
Moe SM, Hack BK, Cummings SA, et al. Role of IL-1 beta and prostaglandins in beta 2-microglobulin-induced bone mineral dissolution. Kidney Int. 1995;47:587–91.
Koch KM. Dialysis-related amyloidosis. Kidney Int. 1992;41:1416–29.
Saito A, Gejyo F. Current clinical aspects of dialysis-related amyloidosis in chronic dialysis patients. Ther Apher Dial. 2006;10:316–20.
Zumrutdal A, Sezer S, Demircan S, et al. Cardiac troponin I and beta 2 microglobulin as risk factors for early-onset atherosclerosis in patients on haemodialysis. Nephrology (Carlton). 2005;10:453–8.
Cheung AK, Rocco MV, Yan G, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17:546–55.
DiRaimondo CR, Casey TT, DiRaimondo CV, et al. Pathologic fractures associated with idiopathic amyloidosis of bone in chronic hemodialysis patients. Nephron. 1986;43:22–7.
Bindi P, Chanard J. Destructive spondyloarthropathy in dialysis patients: an overview. Nephron. 1990;55:104–9.
Kuntz D, Naveau B, Bardin T, et al. Destructive spondylarthropathy in hemodialyzed patients. A new syndrome. Arthritis Rheum. 1984;27:369–75.
Saijo Y, Utsugi M, Yoshioka E, et al. Relationship of beta2-microglobulin to arterial stiffness in Japanese subjects. Hypertens Res. 2005;28:505–11.
Wilson AM, Kimura E, Harada RK, et al. Beta2-microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation. 2007;116:1396–403.
McCarthy JT, Williams AW, Johnson WJ. Serum beta 2-microglobulin concentration in dialysis patients: importance of intrinsic renal function. J Lab Clin Med. 1994;123:495–505.
Locatelli F, Mastrangelo F, Redaelli B, et al. Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. The Italian Cooperative Dialysis Study Group. Kidney Int. 1996;50:1293–302.
Koda Y, Nishi S, Miyazaki S, et al. Switch from conventional to high-flux membrane reduces the risk of carpal tunnel syndrome and mortality of hemodialysis patients. Kidney Int. 1997;52:1096–101.
Locatelli F, Marcelli D, Conte F, et al. Comparison of mortality in ESRD patients on convective and diffusive extracorporeal treatments. The Registro Lombardo Dialisi E Trapianto. Kidney Int. 1999;55:286–93.
Ward RA, Greene T, Hartmann B, et al. Resistance to intercompartmental mass transfer limits beta2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int. 2006;69:1431–7.
Eloot S, Van Biesen W, Dhondt A, et al. Impact of hemodialysis duration on the removal of uremic retention solutes. Kidney Int. 2008;73:765–70.
Skroeder NR, Jacobson SH, Holmquist B, et al. Beta 2-microglobulin generation and removal in long slow and short fast hemodialysis. Am J Kidney Dis. 1993;21:519–26.
Raj DS, Ouwendyk M, Francoeur R, et al. Beta(2)-microglobulin kinetics in nocturnal haemodialysis. Nephrol Dial Transplant. 2000;15:58–64.
Stamopoulos D, Bouziotis P, Benaki D, et al. Nanobiotechnology for the prevention of dialysis-related amyloidosis. Ther Apher Dial. 2009;13:34–41.
Campistol JM. Dialysis-related amyloidosis after renal transplantation. Semin Dial. 2001;14:99–102.
Mourad G, Argiles A. Renal transplantation relieves the symptoms but does not reverse beta 2-microglobulin amyloidosis. J Am Soc Nephrol. 1996;7:798–804.
Bleibel W, Hazar B, Herman R. A case report comparing various radiological tests in the diagnosis of calcific uremic arteriolopathy. Am J Kidney Dis. 2006;48:659–61.
Qunibi WY. Consequences of hyperphosphatemia in patients with end-stage renal disease (ESRD). Kidney Int. 2004;66:S8–12.
Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210–7.
Brandenburg VM, Cozzolino M, Ketteler M. Calciphylaxis: a still unmet challenge. J Nephrol. 2011;24:142–8.
Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139–43.
Au S, Crawford RI. Three-dimensional analysis of a calciphylaxis plaque: clues to pathogenesis. J Am Acad Dermatol. 2002;47:53–7.
Angelis M, Wong LL, Myers SA, et al. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122:1083–9. discussion 9–90.
Mason D, Best SD. Calcific uremic arteriolopathy: contemporary pharmacotherapy. Adv Chronic Kidney Dis. 2010;17:428–38.
Budisavljevic MN, Cheek D, Ploth DW. Calciphylaxis in chronic renal failure. J Am Soc Nephrol. 1996;7:978–82.
Mazhar AR, Johnson RJ, Gillen D, et al. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001;60:324–32.
Rogers NM, Teubner DJ, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150–7.
Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268–70.
Selye H, Gabbiani G, Strebel R. Sensitization to calciphylaxis by endogenous parathyroid hormone. Endocrinology. 1962;71:554–8.
Janigan DT, Hirsch DJ, Klassen GA, et al. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis. 2000;35:588–97.
Sowers KM, Hayden MR. Calcific uremic arteriolopathy: pathophysiology, reactive oxygen species and therapeutic approaches. Oxid Med Cell Longev. 2010;3:109–21.
Moe SM. Calcific uremic arteriolopathy: A new look at an old disorder. NephSAP. 2004;3:77–83.
Hayden MR, Goldsmith DJ. Sodium thiosulfate: new hope for the treatment of calciphylaxis. Semin Dial. 2010;23:258–62.
Krueger T, Westenfeld R, Schurgers L, et al. Coagulation meets calcification: the vitamin K system. Int J Artif Organs. 2009;32:67–74.
Franks AG Jr. Skin manifestations of internal disease. Med Clin North Am. 2009;93:1265–82.
Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569–79.
Rogers NM, Coates PT. Calcific uraemic arteriolopathy: an update. Curr Opin Nephrol Hypertens. 2008;17:629–34.
Rogers NM, Chang SH, Tuebner DJ, et al. Hyperbaric oxygen as effective adjuvant therapy in the treatment of distal calcific uraemic arteriolopathy. NDT Plus. 2008;4:244–9.
Naik BJ, Lynch DJ, Slavcheva EG, et al. Calciphylaxis: medical and surgical management of chronic extensive wounds in a renal dialysis population. Plast Reconstr Surg. 2004;113:304–12.
Girotto JA, Harmon JW, Ratner LE, et al. Parathyroidectomy promotes wound healing and prolongs survival in patients with calciphylaxis from secondary hyperparathyroidism. Surgery. 2001;130:645–50, discussion 50–1.
Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954–62.
Schlieper G, Brandenburg V, Ketteler M, et al. Sodium thiosulfate in the treatment of calcific uremic arteriolopathy. Nat Rev Nephrol. 2009;5:539–43.
Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104–8.
Adams JE. Dialysis bone disease. Semin Dial. 2002;15(4):277–89.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morrow, B., Qunibi, W. Specific Bone and Mineral Disorders in Patients with Chronic Kidney Disease. Clinic Rev Bone Miner Metab 10, 184–208 (2012). https://doi.org/10.1007/s12018-011-9114-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-011-9114-6