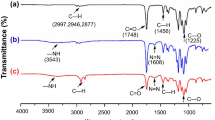
Abstract
An array of quaternary ammonium functionalized-polyhedral oligomeric silsesquioxane (Q-POSS) compounds with different alkyl chain lengths and counter ions were synthesized using a two-step process. First, octasilane POSS was functionalized with dimethylamino groups by hydrosilylation with allyldimethylamine. Next, partial quaternization of the tertiaryamino-functional POSS was achieved using an alkyl halide to produce the Q-POSS. Alkyl chain length of the Q-POSS compounds varied from –C12H25 to –C18H37 and the counter ions varied between chlorine, bromine, and iodine. Moisture-cured polysiloxane coatings were prepared by dispersing Q-POSS molecules into a solution blend of silanol-terminated polydimethylsiloxane, methylacetoxysilane, and a catalyst. To evaluate the utility of the Q-POSS molecules as a broad-spectrum antimicrobial additive, the antimicrobial activity of the coatings toward the Gram-negative bacterium, Escherichia coli, the Gram-positive bacterium, Staphylococcus aureus, and the opportunistic fungal pathogen, Candida albicans, was determined using an agar plating method. The results obtained showed that both the composition of the Q-POSS and the composition of the polysiloxane matrix affected antimicrobial properties. Compositions were identified that inhibited the growth of all three microorganisms on the coating surface. Surface Raman spectroscopic analysis was performed on selected set of coatings to understand the relative concentration of Q-POSS molecules at the coating surface.








Similar content being viewed by others
References
Lligadas, G, Ronda, JC, Galia, M, Cadiz, V, “Bionanocomposites from Renewable Resources: Epoxidized Linseed Oil-Polyhedral Oligomeric Silsesquioxanes Hybrid Materials.” Biomacromolecules, 7 3521–3526 (2006)
Seino, M, Hayakawa, T, Ishida, Y, Kakimoto, M, “Synthesis and Characterization of Crystalline Hyperbranched Polysiloxysilane with POSS Groups at the Terminal Position.” Macromolecules, 39 8892–8894 (2006)
Pellice, SA, Fasce, DP, Williams, RJ, “Properties of Epoxy Networks Derived from the Reaction of Diglycidyl Ether of Bisphenol A with Polyhedral Oligomeric Silsesquioxanes Bearing OH-Functionalized Organic Substituents.” J. Polym. Sci., B: Polym. Phys., 41 1451–1461 (2003)
Huang, JC, He, CB, Xiao, Y, Mya, KY, Dai, J, Siow, YP, “Polyimide/POSS Nanocomposites: Interfacial Interaction, Thermal Properties and Mechanical Properties.” Polymer, 44 4491–4499 (2003)
Xu, HY, Kuo, SW, Lee, JY, Chang, FC, “Glass Transition Temperatures of Poly(hydroxystyrene-co-vinylpyrrolidone-co-isobutylstyryl polyhedral oligosilsesquioxanes).” Polymer, 43 5117–5124 (2002)
Fu, BX, Namani, M, Lee, A, “Influence of Phenyltrisilanol Polyhedral Silsesquioxane on Properties of Epoxy Network Glasses.” Polymer, 44 7739–7747 (2003)
Laine, RM, Choi, J, Lee, I, “Organic-Inorganic Nanocomposites with Completely Defined Interfacial Interactions.” Adv. Mater., 13 800–803 (2001)
Choi, J, Harcup, J, Yee, AF, Zhu, Q, Laine, RM, “Organic/Inorganic Hybrid Composites from Cubic Silsesquioxanes.” J. Am. Chem. Soc., 123 11420–11430 (2001)
Tamaki, R, Tanaka, Y, Asuncion, MZ, Choi, J, Laine, RM, “Octa(aminophenyl)silsesquioxane as a Nanoconstruction Site.” J. Am. Chem. Soc., 123 12416–12417 (2001)
Zhang, C, Babonneau, F, Bonhomme, C, Laine, RM, Soles, CL, Hristov, HA, Yee, AF, “Highly Porous Polyhedral Silsesquioxane Polymers. Synthesis and Characterization.” J. Am. Chem. Soc., 120 8380–8391 (1998)
Russel, AD, “The Mechanism of Action of Some Antibacterial Agents.” Prog. Med. Chem., 6 135–199 (1969)
Sauvet, G, Fortuniak, W, Kazmierski, K, Chojnowski, J, “Amphiphilic Block and Statistical Siloxane Copolymers with Antimicrobial Activity.” J. Polym. Sci., A: Polym. Chem., 41 2939–2948 (2003)
Sauvet, G, Dupond, S, Kazmierski, K, Chojnowski, J, “Biocidal Polymers Active by Contact. V. Synthesis of Polysiloxanes with Biocidal Activity.” J. Appl. Polym. Sci., 75 1005–1012 (2000)
Davies, A, Bentley, M, Field, BS, “Comparison of the Action of Vantocil, Cetrimide, and Chlorhexidine on Escherichia coli and Its Spheroplasts and the Protoplasts of Gram-Positive Bacteria.” J. Appl. Bacteriol., 31 448–452 (1968)
de Brabander-van den Berg, EMM, Meijer, EW, “Poly(propylenimine) Dendrimers: Large-Scale Synthesis via Heterogeneously Catalyzed Hydrogenation.” Angew. Chem. Int. Ed. Engl., 32 1308–1311 (1993)
Gottenbos, B, van der Mei, HC, Klatter, F, Nieuwenhuis, P, Busscher, HJ, “In Vitro and In Vivo Antimicrobial Activity of Covalently Coupled Quaternary Ammonium Silane Coatings on Silicone Rubber.” Biomaterials, 23 (6) 1417–1423 (2002)
Gottenbos, B, Busscher, HJ, van der Mei, HC, Nieuwenhuis, P, “Pathogenesis and Prevention of Biomaterial Centered Infections.” J. Mater. Sci.: Mater. Med., 13 (8) 717–722 (2002)
Dizman, B, Elasri, MO, Mathias, LJ, “Synthesis and Antimicrobial Activities of New Water-Soluble Bis-Quaternary Ammonium Methacrylate Polymers.” J. Appl. Polym. Sci., 94 635–642 (2004)
Chen, CZ, Beck Tan, NC, Cooper, SL, “Incorporation of Dimethyldodecylammonium Chloride Functionalities onto Poly(propylene imine) Dendrimers Significantly Enhances their Antibacterial Properties.” J. Chem. Soc., Chem. Commun., 1585–1586 (1999)
Chojnowski, J, Fortuniak, W, Rosciszewski, P, Werel, W, Łukasiak, J, Kamysz, W, Hałasa, R, “Polysilsesquioxanes and Oligosilsesquioxanes Substituted by Alkylammonium Salts as Antibacterial Biocides.” J. Inorg. Organomet. Polym. Mater., 16 (3) 219–230 (2006)
Majumdar, P, Lee, E, Gubbins, N, Stafslien, SJ, Daniels, J, Thorson, CJ, Chisholm, BJ, “Synthesis and Antimicrobial Activity of Quaternary Ammonium-Functionalized POSS (Q-POSS) and Polysiloxane Coatings Containing Q-POSS.” Polymer, 50 1124–1133 (2009)
Gristina, AG, “Biomaterial-Centered Infection: Microbial Adhesion Versus Tissue Integration.” Science, 237 1588–1595 (1987)
Costerton, JW, Stewart, PS, Greenberg, EP, “Bacterial Biofilms: A Common Cause of Persistent Infections.” Science, 284 1318–1322 (1999)
Copello, GJ, Teves, S, Degrossi, J, D’Aquino, M, Desimone, MF, Diaz, LE, “Antimicrobial Activity on Glass Materials Subject to Disinfectant Xerogel Coating.” J. Ind. Microbiol. Biotechnol., 33 343–348 (2006)
Neumann, D, Fisher, M, Tran, L, Matisons, JG, “Synthesis and Characterization of an Isocyanate Functionalized Polyhedral Oligosilsesquioxane and the Subsequent Formation of an Organic-Inorganic Hybrid Polyurethane.” J. Am. Chem. Soc., 124 (47) 13998–13999 (2002)
Gilbert, P, Al-Taae, A, “Antimicrobial Activity of Some Alkyltrimethylammonium Bromides.” Lett. Appl. Microbiol., 1 101–104 (1985)
Kourai, H, Horie, T, Takeichi, K, Shibasaki, IJ, “The Antimicrobial Characteristics of Quaternary Ammonium Salts and their Alkyl Chain Length.” Antibact. Antifung. Agents, 8 9–17 (1980)
Chen, CZ, Beck-Tan, NC, Dhurjati, P, van Dyk, TK, LaRossa, RA, Copper, SL, “Quaternary Ammonium Functionalized Poly(propylene imine) Dendrimers as Effective Antimicrobials: Structure-Activity Studies.” Biomacromolecules, 1 473–480 (2000)
Juergensen, L, Busnarda, J, Caux, PY, Kent, RA, “Fate, Behavior, and Aquatic Toxicity of the Fungicide DDAC in the Canadian Environment.” Environ. Toxicol., 15 (3) 174–200 (2000)
Veres, M, Koos, M, Toth, S, Fuele, M, Pocsik, I, Toth, A, Mohai, M, Bertoti, I, “Characterisation of a-C:H and Oxygen-Containing Si:C:H Films by Raman Spectroscopy and XPS.” Diam. Relat. Mater., 14 (3–7) 1051–1056 (2005)
Cheong, CUA, Stair, PC, “In Situ Measurements of Lubricant Temperature and Pressure at a Sliding Contact.” J. Phys. Chem. C, 111 (30) 11314–11319 (2007)
Leary, TJC, Levin, IW, “Effects of Solvent on Biomembrane Structure: Raman Spectroscopic Investigation of Dipalmitoylphosphatidylcholine Dispersed in N-ethylammonium Nitrate.” J. Phys. Chem., 88 (18) 4074–4078 (1984)
Leary, TJC, Levin, IW, “Raman Spectroscopic Study of the Melting Behavior of Anhydrous Dipalmitoylphosphatidylcholine Bilayers.” J. Phys. Chem., 88 (9) 1709–1796 (1984)
Kinowski, C, Bouazaoui, M, Bechara, R, Hench, LL, Nedelec, JM, Turrell, S, “Kinetics of Densification of Porous Silica Gels: A Structural and Textural Study.” J. Noncryst. Solids, 291 (3) 143–152 (2001)
Awazu, K, “Fluorinated Silica Glass Ablated with ArF Excimer Laser at Low Fluence.” J. Noncryst. Solids, 353 (2) 215–217 (2007)
Takala, M, Karttunen, M, Salovaara, P, Kortet, S, Kannus, K, Kalliohaka, T, “Dielectric Properties of Nanostructured Polypropylene- Polyhedral Oligomeric Silsesquioxane Compounds.” IEEE Trans. Dielectr. Electr. Insulation, 15 (1) 40–51 (2008)
Dutta, PK, Shieh, DC, Puri, M, “Raman Spectroscopic Study of the Synthesis of Zeolite Y.” J. Phys. Chem., 91 (9) 2332–2336 (1987)
Acknowledgment
The authors acknowledge financial support from the Office of Naval Research under grants N00014-05-1-0822 and N00014-06-1-0952.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented at the 2009 CoatingsTech Conference, sponsored by NPCA/FSCT, April 28–29, 2009, in Indianapolis, IN.
Appendix 1: Synthesis details for Q-POSS compounds
Appendix 1: Synthesis details for Q-POSS compounds
Synthesis of tertiaryamino-functional POSS
In a 100-mL round-bottom flask equipped with a nitrogen inlet, condenser, and temperature controller, 2.00 g of octasilane POSS (1.96 mmol) and 1.75 g of allyldimethylamine (20.55 mmol) were dissolved in 50 mL of THF. Once dissolved, 180 μL of Karstedt’s catalyst was added to the reaction mixture and the reaction mixture was refluxed for 48 h. Completion of the reaction was confirmed using proton nuclear magnetic resonance spectroscopy (1H NMR) by observing the disappearance of the Si–H peak at δ 4.7 ppm. After completion of the hydrosilylation reaction, excess allyldimethylamine was removed under reduced pressure. Allydimethylamine removal was confirmed by the absence of the 1H NMR (in CDCl3) peaks at δ 5.72 ppm (–N–CH2–CH=) and 5.01 ppm (−N−CH2−CH=CH 2 ). 29Si NMR displayed a singlet at δ 14.9 ppm, corresponding to the M-type silicon, and another singlet at δ −107.3 ppm, corresponding to the Q-type silicon of the POSS core. The presence of only two singlets in the 29Si NMR spectrum confirmed that the cubic structure of POSS remained intact during the reaction. 1H NMR (in CDCl3) peaks are: δ 0.02–0.03 ppm (Si–CH 3 ), δ 0.45–0.48 ppm [Si–CH 2 –CH2–CH2–N and Si–CH(CH3)–CH2–N], δ 0.88 ppm [Si–CH(CH 3 )–CH2–N], δ 1.39 ppm (Si–CH2–CH 2 –CH2–N), and δ 2.09–2.22 ppm [−CH 2 –N(CH 3 )2]. The proportion of α-isomer was 20%.
Synthesis of Q-POSS (octadecyldimethylammoniumiodide-POSS, Q-18-I)
In a 20-mL glass vial containing a magnetic stir bar, 2.00 g of the tertiaryamino-functional POSS (9.4 × 10−3 moles of tertiary amine functional groups) was mixed with 1.43 g of 1-iodooctadecane (3.76 × 10−3 moles) and the quaternization reaction was carried out at 50°C for 48 h. A substantial increase in viscosity was observed as a result of the reaction. Using 1H NMR (in CDCl3), new peaks appeared at δ 3.53 ppm (–N+–CH 2 –) and 3.27 ppm [(–N+–(CH 3 )2] due to quaternization. 1H NMR (in CDCl3) peaks are: δ 0.01–0.09 ppm (Si–CH 3 ), δ 0.45–0.55 ppm [Si–CH 2 –CH2–CH2–N+, Si–CH(CH3)–CH2–N+, Si–CH 2 –CH2–CH2–N, and Si–CH(CH3)–CH2–N], δ 0.86 ppm [–(CH2)17–CH 3 ], δ 0.93 ppm [Si–CH(CH 3 )–CH2–N+ and Si–CH(CH 3 )–CH2–N], δ 1.22 ppm [–CH2–CH2–(CH 2 )15–CH3], δ 1.38 ppm (Si–CH2–CH 2 –CH2–N and Si–CH2–CH 2 –CH2–N+), δ 1.71 ppm [N+–CH2–CH 2 –(CH2)15–CH3], δ 2.09–2.22 ppm [–CH 2 –N(CH 3 )2], δ 3.27 ppm [(–N+–(CH 3 )2], and δ 3.53 ppm (–N+–CH 2 –). The extent of quaternization was 34.3 mol%. The Q-POSS was diluted with THF to produce 5 and 50 wt% solution.
Synthesis of Q-POSS (hexadecyldimethylammoniumiodide-POSS, Q-16-I)
In a 20-mL glass vial containing a magnetic stir bar, 2.00 g of the tertiaryamino-functional POSS (9.4 × 10−3 moles of tertiary amine functional groups) was mixed with 1.32 g of 1-iodohexadecane (3.76 × 10−3 moles) and the quaternization reaction was carried out at 50°C for 48 h. A substantial increase in viscosity was observed as a result of the reaction. Using 1H NMR (in CDCl3), new peaks appeared at δ 3.54 ppm (–N+–CH 2 –) and 3.28 ppm [(–N+–(CH 3 )2] due to quaternization. 1H NMR (in CDCl3) peaks are: δ 0.03–0.11 ppm (Si–CH 3 ), δ 0.44–0.56 ppm [Si–CH 2 –CH2–CH2–N+, Si–CH(CH3)–CH2–N+, Si–CH 2 –CH2–CH2–N, and Si–CH(CH3)–CH2–N], δ 0.85 ppm [–(CH2)15–CH 3 ], δ 0.93 ppm [Si–CH(CH 3 )–CH2–N+ and Si–CH(CH 3 )–CH2–N], δ 1.23 ppm [–CH2–CH2–(CH 2 )13–CH3], δ 1.38 ppm (Si–CH2–CH 2 –CH2–N and Si–CH2–CH 2 –CH2–N+), δ 1.72 ppm [N+–CH2–CH 2 –(CH2)13–CH3], δ 2.09–2.22 ppm [–CH 2 –N(CH 3 )2], δ 3.28 ppm [(–N+–(CH 3 )2], and δ 3.54 ppm (–N+–CH 2 –). The extent of quaternization was 37.3 mol%. The Q-POSS was diluted with THF to produce 5 and 50 wt% solution.
Synthesis of Q-POSS (dodecyldimethylammoniumiodide-POSS, Q-12-I)
In a 20-mL glass vial containing a magnetic stir bar, 2.00 g of the tertiaryamino-functional POSS (9.4 × 10−3 moles of tertiary amine functional groups) was mixed with 1.11 g of 1-iodododecane (3.76 × 10−3 moles) and the quaternization reaction was carried out at 50°C for 48 h. A substantial increase in viscosity was observed as a result of the reaction. Using 1H NMR (in CDCl3), new peaks appeared at δ 3.51 ppm (–N+–CH 2 –) and 3.25 ppm [(–N+–(CH 3 )2] due to quaternization. 1H NMR (in CDCl3) peaks are: δ 0.03–0.08 ppm (Si–CH 3 ), δ 0.44–0.53 ppm [Si–CH 2 –CH2–CH2–N+, Si–CH(CH3)–CH2–N+, Si–CH 2 –CH2–CH2–N, and Si–CH(CH3)–CH2–N], δ 0.82 ppm [–(CH2)11–CH 3 ], δ 0.93 ppm [Si–CH(CH 3 )–CH2–N+ and Si–CH(CH 3 )–CH2–N], δ 1.20 ppm [–CH2–CH2–(CH 2 )9–CH3], δ 1.38 ppm (Si–CH2–CH 2 –CH2–N and Si–CH2–CH 2 –CH2–N+), δ 1.69 ppm [N+–CH2–CH 2 –(CH2)13–CH3], δ 2.09–2.22 ppm [–CH 2 –N(CH 3 )2], δ 3.25 ppm [(–N+–(CH 3 )2], and δ 3.51 ppm (–N+–CH 2 –). The extent of quaternization was 34.3 mol%. The Q-POSS was diluted with THF to produce 5 and 50 wt% solution.
Synthesis of Q-POSS (octadecyldimethylammoniumbromide-POSS Q-18-Br)
In a 20-mL glass vial containing a magnetic stir bar, 2.00 g of the tertiaryamino-functional POSS (9.4 × 10−3 moles of tertiary amine functional groups) was mixed with 1.26 g of 1-bromooctadecane (3.76 × 10−3 moles) and the quaternization reaction was carried out at 50°C for 48 h. A substantial increase in viscosity was observed as a result of the reaction. Using 1H NMR (in CDCl3), new peaks appeared at δ 3.53 ppm (–N+–CH 2 –) and 3.30 ppm [(–N+–(CH 3 )2] due to quaternization. 1H NMR (in CDCl3) peaks are: δ 0.03–0.11 ppm (Si–CH 3 ), δ 0.45–0.56 ppm [Si–CH 2 –CH2–CH2–N+, Si–CH(CH3)–CH2–N+, Si–CH 2 –CH2–CH2–N, and Si–CH(CH3)–CH2–N], δ 0.85 ppm [–(CH2)17–CH 3 ], δ 0.96 ppm [Si–CH(CH 3 )–CH2–N+ and Si–CH(CH 3 )–CH2–N], δ 1.23 ppm [–CH2–CH2–(CH 2 )15–CH3], δ 1.45 ppm (Si–CH2–CH 2 –CH2–N and Si–CH2–CH 2 –CH2–N+), δ 1.68 ppm [N+–CH2–CH 2 –(CH2)15–CH3], δ 2.09–2.22 ppm [–CH 2 –N(CH 3 )2], δ 3.30 ppm [(–N+–(CH 3 )2], and δ 3.53 ppm (–N+–CH 2 –). The extent of quaternization was 27.2 mol%. The Q-POSS was diluted with THF to produce 5 and 50 wt% solution.
Synthesis of Q-POSS (hexadecyldimethylammoniumbromide-POSS, Q-16-Br)
In a 20-mL glass vial containing a magnetic stir bar, 2.00 g of the tertiaryamino-functional POSS (9.4 × 10−3 moles of tertiary amine functional groups) was mixed with 1.15 g of 1-bromohexadecane (3.76 × 10−3 moles) and the quaternization reaction was carried out at 50°C for 48 h. A substantial increase in viscosity was observed as a result of the reaction. Using 1H NMR (in CDCl3), new peaks appeared at δ 3.52 ppm (–N+–CH 2 –) and 3.28 ppm [(–N+–(CH 3 )2] due to quaternization. 1H NMR (in CDCl3) peaks are: δ 0.03–0.10 ppm (Si–CH 3 ), δ 0.44–0.55 ppm [Si–CH 2 –CH2–CH2–N+, Si–CH(CH3)–CH2–N+, Si–CH 2 –CH2–CH2–N, and Si–CH(CH3)–CH2–N], δ 0.84 ppm [–(CH2)15–CH 3 ], δ 0.95 ppm [Si–CH(CH 3 )–CH2–N+ and Si–CH(CH 3 )–CH2–N], δ 1.21 ppm [–CH2–CH2–(CH 2 )13–CH3], δ 1.45 ppm (Si–CH2–CH 2 –CH2–N and Si–CH2–CH 2 –CH2–N+), δ 1.68 ppm [N+–CH2–CH 2 –(CH2)13–CH3], δ 2.09–2.22 ppm [–CH 2 –N(CH 3 )2], δ 3.28 ppm [(–N+–(CH 3 )2], and δ 3.52 ppm (–N+–CH 2 –). The extent of quaternization was 34.5 mol%. The Q-POSS was diluted with THF to produce 5 and 50 wt% solution.
Synthesis of Q-POSS (dodecyldimethylammoniumbromide-POSS Q-12-Br)
In a 20-mL glass vial containing a magnetic stir bar, 2.00 g of the tertiaryamino-functional POSS (9.4 × 10−3 moles of tertiary amine functional groups) was mixed with 0.94 g of 1-bromododecane (3.76 × 10−3 moles) and the quaternization reaction was carried out at 50°C for 48 h. A substantial increase in viscosity was observed as a result of the reaction. Using 1H NMR (in CDCl3), new peaks appeared at δ 3.51 ppm (–N+–CH 2 –) and 3.28 ppm [(–N+–(CH 3 )2] due to quaternization. 1H NMR (in CDCl3) peaks are: δ 0.03–0.09 ppm (Si–CH 3 ), δ 0.44–0.54 ppm [Si–CH 2 –CH2–CH2–N+, Si–CH(CH3)–CH2–N+, Si–CH 2 –CH2–CH2–N, and Si–CH(CH3)–CH2–N], δ 0.83 ppm [–(CH2)11–CH 3 ], δ 0.94 ppm [Si–CH(CH 3 )–CH2–N+ and Si–CH(CH 3 )–CH2–N], δ 1.21 ppm [–CH2–CH2–(CH 2 )9–CH3], δ 1.45 ppm (Si–CH2–CH 2 –CH2–N and Si–CH2–CH 2 –CH2–N+), δ 1.68 ppm [N+–CH2–CH 2 –(CH2)13–CH3], δ 2.09–2.22 ppm [–CH 2 –N(CH 3 )2], δ 3.28 ppm [(–N+–(CH 3 )2], and δ 3.51 ppm (–N+–CH 2 –). The extent of quaternization was 34.4 mol%. The Q-POSS was diluted with THF to produce 5 and 50 wt% solution.
Synthesis of Q-POSS (octadecyldimethylammoniumchloride-POSS, Q-18-Cl)
In a 20-mL glass vial containing a magnetic stir bar, 2.00 g of the tertiaryamino-functional POSS (9.4 × 10−3 moles of tertiary amine functional groups) was mixed with 1.09 g of 1-chlorooctadecane (3.76 × 10−3 moles) and the quaternization reaction was carried out at 110°C for 48 h. A substantial increase in viscosity was observed as a result of the reaction. Using 1H NMR (in CDCl3), new peaks appeared at δ 3.40 ppm (–N+–CH 2 –) and 3.31 ppm [(–N+–(CH 3 )2] due to quaternization. 1H NMR (in CDCl3) peaks are: δ 0.03–0.09 ppm (Si–CH 3 ), δ 0.45–0.54 ppm [Si–CH 2 –CH2–CH2–N+, Si–CH(CH3)–CH2–N+, Si–CH 2 –CH2–CH2–N, and Si–CH(CH3)–CH2–N], δ 0.84 ppm [–(CH2)17–CH 3 ], δ 0.95 ppm [Si–CH(CH 3 )–CH2–N+ and Si–CH(CH 3 )–CH2–N], δ 1.22 ppm [–CH2–CH2–(CH 2 )15–CH3], δ 1.45 ppm (Si–CH2–CH 2 –CH2–N and Si–CH2–CH 2 –CH2–N+), δ 1.67 ppm [N+–CH2–CH 2 –(CH2)15–CH3], δ 2.13–2.22 ppm [–CH 2 –N(CH 3 )2], δ 3.31 ppm [(–N+–(CH 3 )2], and δ 3.40 ppm (–N+–CH 2 –). The extent of quaternization was 27.2 mol%. The Q-POSS was diluted with THF to produce 5 and 50 wt% solution.
Synthesis of Q-POSS (hexadecyldimethylammoniumchloride-POSS, Q-16-Cl)
In a 20-mL glass vial containing a magnetic stir bar, 2.00 g of the tertiaryamino-functional POSS (9.4 × 10−3 moles of tertiary amine functional groups) was mixed with 0.98 g of 1-chlorohexadecane (3.76 × 10−3 moles) and the quaternization reaction was carried out at 110°C for 48 h. A substantial increase in viscosity was observed as a result of the reaction. Using 1H NMR (in CDCl3), new peaks appeared at δ 3.40 ppm (–N+–CH 2 –) and 3.32 ppm [(–N+–(CH 3 )2] due to quaternization. 1H NMR (in CDCl3) peaks are: δ 0.03–0.09 ppm (Si–CH 3 ), δ 0.44–0.53 ppm [Si–CH 2 –CH2–CH2–N+, Si–CH(CH3)–CH2–N+, Si–CH 2 –CH2–CH2–N, and Si–CH(CH3)–CH2–N], δ 0.84 ppm [–(CH2)15–CH 3 ], δ 0.95 ppm [Si–CH(CH 3 )–CH2–N+ and Si–CH(CH 3 )–CH2–N], δ 1.22 ppm [–CH2–CH2–(CH 2 )13–CH3], δ 1.44 ppm (Si–CH2–CH 2 –CH2–N and Si–CH2–CH 2 –CH2–N+), δ 1.67 ppm [N+–CH2–CH 2 –(CH2)13–CH3], δ 2.13–2.22 ppm [–CH 2 –N(CH 3 )2], δ 3.32 ppm [(–N+–(CH 3 )2], and δ 3.40 ppm (–N+–CH 2 –). The extent of quaternization was 28.4 mol%. The Q-POSS was diluted with THF to produce 5 and 50 wt% solution.
Synthesis of Q-POSS (dodecyldimethylammoniumchloride-POSS, Q-12-Cl)
In a 20-mL glass vial containing a magnetic stir bar, 2.00 g of the tertiaryamino-functional POSS (9.4 × 10−3 moles of tertiary amine functional groups) was mixed with 0.94 g of 1-bromododecane (3.76 × 10−3 moles) and the quaternization reaction was carried out at 110°C for 48 h. A substantial increase in viscosity was observed as a result of the reaction. Using 1H NMR (in CDCl3), new peaks appeared at δ 3.40 ppm (–N+–CH 2 –) and 3.32 ppm [(–N+–(CH 3 )2] due to quaternization. 1H NMR (in CDCl3) peaks are: δ 0.03–0.11 ppm (Si–CH 3 ), δ 0.44–0.56 ppm [Si–CH 2 –CH2–CH2–N+, Si–CH(CH3)–CH2–N+, Si–CH 2 –CH2–CH2–N, and Si–CH(CH3)–CH2–N], δ 0.85 ppm [–(CH2)11–CH 3 ], δ 0.96 ppm [Si–CH(CH 3 )–CH2–N+ and Si–CH(CH 3 )–CH2–N], δ 1.22 ppm [–CH2–CH2–(CH 2 )9–CH3], δ 1.43 ppm (Si–CH2–CH 2 –CH2–N and Si–CH2–CH 2 –CH2–N+), δ 1.68 ppm [N+–CH2–CH 2 –(CH2)13–CH3], δ 2.14–2.22 ppm [–CH 2 –N(CH 3 )2], δ 3.32 ppm [(–N+–(CH 3 )2], and δ 3.40 ppm (–N+–CH 2 –). The extent of quaternization was 28.9 mol%. The Q-POSS was diluted with THF to produce 5 and 50 wt% solution.
Rights and permissions
About this article
Cite this article
Majumdar, P., He, J., Lee, E. et al. Antimicrobial activity of polysiloxane coatings containing quaternary ammonium-functionalized polyhedral oligomeric silsesquioxane. J Coat Technol Res 7, 455–467 (2010). https://doi.org/10.1007/s11998-009-9197-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-009-9197-x