Opinion statement
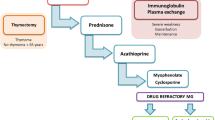
Treatment for myasthenia gravis should be individualized to each patient based on the clinical characteristics of myasthenia including the distribution, duration, and severity of weakness and resulting functional impairment; the risks for treatment complications related to age, gender, and medical comorbidities; and the presence of thymoma. Acetylcholinesterase inhibitors provide temporary, symptomatic treatment for all forms of myasthenia gravis. Immune modulators address the underlying autoimmune process in myasthenia gravis, but are associated with potential complications and side effects. Most patients with generalized myasthenia who have significant weakness beyond the ocular muscles and who remain symptomatic, despite treatment with cholinesterase inhibitors, are candidates for immune modulation. Although corticosteroids are effective for long-term immune modulation in myasthenia gravis, several more contemporary immunomodulators including azathioprine, cyclosporine, and mycophenolate mofetil have shown efficacy in myasthenia gravis and are used increasingly as first-line treatments and as steroid-sparing agents. Plasma exchange is used to achieve rapid improvement in patients with myasthenic crisis or exacerbation, to improve strength before a surgical procedure or thymectomy, and to minimize steroid-induced exacerbation in patients with oropharyngeal or respiratory muscle weakness. Intravenous immunoglobulin represents an alternative to plasma exchange in patients requiring relatively rapid short-term improvement in the setting of poor venous access. Because of a lack of controlled trials, the role of thymectomy in nonthymomatous myasthenia gravis is unclear, although evidence suggests that thymectomy increases the probability for myasthenic remission or improvement.
Similar content being viewed by others
References and Recommended Reading
Grob D: Natural history of myasthenia gravis. In Myasthenia Gravis and Myasthenic Disorders. Edited by Engel AG. New York: Oxford University Press; 1999:131–154.
Fink ME: Treatment of the critically ill patient with myasthenia gravis. In Neurological and Neurosurgical Intensive Care, 3rd Edition. Edited by Ropper AH. New York: Raven Press, 1993:351–362.
Pascuzzi RM: Medications and myasthenia gravis: a reference for health care professionals. http://www.myasthenia.org/drugs/reference.htm. Accessed October 1, 2004. This is a complete and updated list of medications increasing weakness in myasthenia gravis.
Namba T, Brunner NG, Grob D: Myasthenia gravis in patients with thymoma, with particular reference to onset after thymectomy. Medicine 1957, 57:411–433.
Sanders DB, Andrews PI, Howard JF, Massey JM: Seronegative myasthenia gravis. Neurology 1997, 48(suppl 5):40S-45S.
Evoli A, Tonali PA, Padua L, et al.: Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003, 126:2304–2311.
Hoch W, McConville J, Helms S, et al.: Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 2001, 7:365–368. This was an initial demonstration of an immunologicallydistinct form of myasthenia gravis with MuSK antibodies.
McConville J, Farrugia ME, Beeson D, et al.: Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol 2004, 55:580–584.
Sanders DB, El-Salem K, Massey JM, et al.: Clinical aspects of MuSK antibody positive seronegative myasthenia gravis (SNMG). Neurology 2003, 60:1978–1980.
Scuderi F, Marino M, Colonna L, et al.: Anti-P110 autoantibodies identify a subtype of “seronegative” myasthenia gravis with prominent oculobulbar involvement. Lab Invest 2002, 82:1139–1146.
Walker MB: Treatment of myasthenia gravis with physostigmine. Lancet 1934, 1:1200–1201.
Oosterhuis HJGH: The natural course of myasthenia gravis: a long term follow up study. J Neurol Neurosurg Psychiatry 1989, 52:1121–1127.
Pascuzzi RM, Coslett HB, Johns TR: Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol 1984, 15:291–298.
Johns TR: Long-term corticosteroid treatment of myasthenia gravis. Ann N Y Acad Sci 1987, 505:568–583.
Miller RG, Milner-Brown HS, Mirka A: Prednisoneinduced worsening of neuromuscular function in myasthenia gravis. Neurology 1986, 36:729–732.
Seybold ME, Drachman DB: Gradually increasing doses of prednisone in myasthenia gravis: reducing the hazards of treatment. N Engl J Med 1974, 290:81–84.
Palace J, Newsom-Davis J, Lecky B: A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Myasthenia Gravis Study Group. Neurology 1998, 50:1778–1783. This is a prospective, controlled, randomized study confirming the efficacy of azathioprine as a steroid sparing agent in myasthenia gravis.
Hohlfeld R, Michels M, Heininger K, et al.: Azathioprine toxicity during long-term immunosuppression of generalized myasthenia gravis. Neurology 1988, 38:258–261.
Kissel JT, Levy RJ, Mendell JR, Griggs RC: Azathioprine toxicity in neuromuscular disease. Neurology 1986, 36:35–39.
Herrllinger U, Weller M, Dichgans J, Melms A: Association of primary central nervous system lymphoma with long-term azathioprine therapy for myasthenia gravis. Ann Neurol 2000, 47:682–683.
Tindall RS, Phillips JT, Rollins JA, et al.: A clinical therapeutic trial of cyclosporine in myasthenia gravis. Ann N Y Acad Sci 1993, 681:539–551.
Ciafaloni E, Nikhar NK, Massey JM, Sanders DB: Retrospective analysis of the use of cyclosporine in myasthenia gravis. Neurology 2000, 55:448–450. This long-term study demonstrated the effectiveness of cyclosporine as a steroid sparing agent and documented side effects.
Myasthenia Gravis Foundation of America Medical/Scientific and Nurses Advisory Boards: Cyclosporine. http://www.myasthenia.org/information/Cyclosporine. htm. Accessed October 1, 2004. This informational brochure contains a complete listing of medication interactions with cyclosporine.
Sollinger HW for the U.S. Renal Transplant Mycophenolate Mofetil Study Group: Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation 1995, 60:225–232.
Chaudhry V, Cornblath DR, Griffin JW, et al.: Mycophenolate mofetil: a safe and promising immunosuppressant in neuromuscular diseases. Neurology 2001, 56:94–96.
Ciafaloni E, Massey JM, Tucker-Lipscomb B, Sanders DB: Mycophenolate mofetil for myasthenia gravis: an open-label pilot study. Neurology 2001, 56:97–99.
Meriggioli MN, Rowin J, Richman JG, Leurgans S: Mycophenolate mofetil for myasthenia gravis: a double-blind, placebo-controlled pilot study. Ann N Y Acad Sci 2003, 998:494–499.
Meriggioli MN, Ciafaloni E, Al-Hayk KA, et al.: Mycophenolate mofetil for myasthenia gravis: an analysis of efficacy, safety, and tolerability. Neurology 2003, 61:1438–1440. This retrospective study of 85 myasthenic patients documents significant improvement with mycophenolate mofetil treatment and few side effects.
Pinching AF, Peters DK, Newsom-Davis J: Remission of myasthenia gravis following plasma exchange. Lancet 1976, 2:1373–1376.
Dau PC, Lindstrom JM, Cassel CK, et al.: Plasmapheresis and immunosuppressive drug therapy in myasthenia gravis. N Engl J Med 1977, 297:1134–1140.
Antozzi C, Gemma M, Regi B, et al.: A short plasma exchange protocol is effective in severe myasthenia gravis. J Neurol 1991, 238:103–107.
NIH Consensus Conference: The utility of therapeutic plasmapheresis for neurological disorders. JAMA 1986, 256:1333–1337.
Gajdos P, Chevret S, Toyka K: Plasma exchange for myasthenia gravis. Cochrane Database of Systematic Reviews 2003; 3. http://www.cochrane.org/cochrane/revabstr/ab002275.htm. Abstract accessed October 1, 2004. This is an evidence-based review of plasma exchange in myasthenia gravis.
Siami GA, Siami FS, Morrow JD, Stone WJ: Cryofiltration aperesis and plasma fractionation causing anaphylactoid reactions in patients receiving angiotensin converting enzyme inhibitors. Therapeutic Apheresis 1997, 1:325–329.
Gajdos P, Chevret S, Clair B, et al.: Myasthenia Gravis Clinical Study Group. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Ann Neurol 1997, 41:789–796.
Qureshi AI, Choudhry MA, Akbar MS, et al.: Plasma exchange versus intravenous immunoglobulin treatment in myasthenic crisis. Neurology 1999, 52:629–632.
Stricker RB, Kwiatkowska BJ, Habis JA, Kiprov DD: Myasthenic crisis. Response to plasmapheresis following failure of intravenous gamma-globulin. Arch Neurol 1993, 50:837–840.
Gajdos P, Chevret S, Toyka K: Intravenous immunoglobulin for myasthenia gravis. Cochrane Database of Systematic Reviews 2003; 3. http://www.cochrane.org/cochrane/revabstr/ab002277.htm. Abstract accessed October 1, 2004. This is an evidence-based review of intravenous immunoglobulin in myasthenia gravis.
Huang C-S, Hsu H-S, Kao K-P, et al.: Intravenous immunoglobulin in the preparation of thymectomy for myasthenia gravis. Acta Neurol Scand 2003, 108:136–138.
Tan E, Hajinazarian M, Bay W, et al.: Acute renal failure resulting from intravenous immunoglobulin therapy. Arch Neurol 1993, 50:137–139.
Caress JB, Cartwright MS, Donofrio PD, Peacock JE: The clinical features of 16 cases of stroke associated with administration of IVIg. Neurology 2003, 60:1822–1824.
Blalock A, Harvey AM, Ford FR, Lilientha Jr JL: The treatment of myasthenia gravis by removal of the thymus gland. Preliminary report. JAMA 1945, 127:1089–1096.
Blalock A: Thymectomy in the treatment of myasthenia gravis. Report of twenty cases. J Thorac Surg 1944, 13:316–339.
Gronseth GS, Barohn RJ: Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000, 55:7–15. This is an evidence-based evaluation of the literature on thymectomy in myasthenia gravis.
Wolfe GI, Kaminski HJ, Jaretzki A, et al.: Development of a thymectomy trial in nonthymomatous myasthenia gravis patients receiving immunosuppressive therapy. Ann N Y Acad Sci 2003, 998:473–480.
Perlo VP, Arnason B, Poskanzer D, et al.: The role of thymectomy in the treatment of myasthenia gravis. Ann N Y Acad Sci 1971, 183:308–315.
Rodriguez M, Gomez MR, Howard FM, Taylor WF: Myasthenia gravis in children: long-term follow-up. Ann Neurol 1983, 13:504–510.
Mulder DG, Graves M, Hermann C: Thymectomy for myasthenia gravis: recent observation and comparisons with past experience. Ann Thorac Surg 1989, 48:551–555.
Monden Y, Nakahara K, Kagotani K, et al.: Effects of preoperative duration of symptoms on patients with myasthenia gravis. Ann Thorac Surg 1984, 38:287–291.
Beghi E, Antozzi C, Batocchi AP, et al.: Prognosis of myasthenia gravis: a multicenter follow-up study of 844 patients. J Neurol Sci 1991, 106:213–220.
Lanska DJ: Indications for thymectomy in myasthenia gravis. Neurology 1990, 40:1828–1829.
Schumm F, Wietholter H, Fateh-Moghadam A, Dichgans J: Thymectomy in myasthenia with pure ocular symptoms. J Neurol Neurosurg Psychiatry 1985, 48:332–337.
Jaretzki III A: Thymectomy for myasthenia gravis: analysis of the controversies regarding technique and results. Neurology 1997, 48(suppl 5):S52-S63.
Jaretzki III A, Steinglass KM, Sonett JR: Thymectomy in the management of myasthenia gravis. Semin Neurol 2004, 24:49–62. This is a contemporary summary of surgical techniques for thymectomy in myasthenia gravis.
Jaretzki III A, Aarli JA, Kaminski HJ, et al.: Preoperative preparation of patients with myasthenia gravis forestalls postoperative respiratory complications after thymectomy. Ann Thorac Surg 2003, 75:1068.
Bulkley GB, Bass KN, Stephenson GR, et al.: Extended cervicomediastinal thymectomy in the integrated management of myasthenia gravis. Ann Surg 1997, 226:324–334.
Evoli A, Di Schino C, Marsili F, Punzi C: Successful treatment of myasthenia gravis with tacrolimus. Muscle Nerve 2002, 25:111–114.
Yoshikawa H, Mabuchi K, Yasukawa Y, et al.: Low-dose tacrolimus for intractable myasthenia gravis. J Clin Neurosci 2002, 9:627–628.
Wylam ME, Anderson PM, Kuntz NL, Rodirguez V: Successful treatment of refractory myasthenia gravis using rituximab: a pediatric case report. J Pediatr 2003, 143:674–677.
Zaja F, Russo D, Fuga G, et al.: Rituximab for myasthenia gravis. Ann N Y Acad Sci 1987, 505:832–835.
Andrews PI: Autoimmune myasthenia gravis in childhood. Semin Neurol 2004, 24:101–110. This is a contemporary review of diagnosis and treatment in juvenile myasthenia gravis.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Juel, V.C., Massey, J.M. Autoimmune myasthenia gravis: Recommendations for treatment and immunologic modulation. Curr Treat Options Neurol 7, 3–14 (2005). https://doi.org/10.1007/s11940-005-0001-7
Issue Date:
DOI: https://doi.org/10.1007/s11940-005-0001-7