Abstract
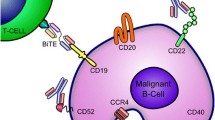
Anaplastic large cell lymphoma (ALCL) is a lymphoid neoplasm characterized by strong and uniform expression of the CD30 antigen on the cell surface. Current standard frontline therapy of ALCL is anthracycline-based combination chemotherapy, usually CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) or CHOP-like regimens. Despite aggressive chemotherapy a significant number of patients relapse. Newer agents and strategies are needed in the management of this challenging disease especially in ALK-negative and high-risk ALK-positive patients who tend to have a poor prognosis. In this review we discuss the different approaches to targeting CD30 including naked antibodies, “enhanced antibodies”, antibody drug–toxin conjugates, radioimmunoconjugates, CD30-ligand–toxin conjugates, bispecific antibodies and T cell-based immune therapies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008.
Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496–504. doi:10.1182/blood-2008-01-134270.
Zinzani PL, Bendandi M, Martelli M, Falini B, Sabattini E, Amadori S, et al. Anaplastic large-cell lymphoma: clinical and prognostic evaluation of 90 adult patients. J Clin Oncol. 1996;14(3):955–62.
Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328(14):1002–6. doi:10.1056/NEJM199304083281404.
Simon A, Peoch M, Casassus P, Deconinck E, Colombat P, Desablens B, et al. Upfront VIP–reinforced–ABVD (VIP–rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS–LTP95. Br J Haematol. 2010;151(2):159–66.
Zelenetz AD, Hamlin P, Kewalramani T, Yahalom J, Nimer S, Moskowitz CH. Ifosfamide, carboplatin, etoposide (ICE)-based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2003;14 suppl 1:i5–10. doi:10.1093/annonc/mdg702.
Velasquez W, Cabanillas F, Salvador P, McLaughlin P, Fridrik M, Tucker S, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP). Blood. 1988;71(1):117–22.
Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, et al. ESHAP – an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12(6):1169–76.
O'Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182–9. doi:10.1200/jco.2010.29.9024.
Piekarz RL, Frye R, Prince HM, Kirschbaum MH, Zain J, Allen SL, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117(22):5827–34. doi:10.1182/blood-2010-10-312603.
Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results from a pivotal, open-label, phase ii study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631–6. doi:10.1200/jco.2011.37.4223.
Dürkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992;68(3):421–7. doi:10.1016/0092-8674(92)90180-k.
Stein H, Mason D, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848–58.
Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85(1):1–14.
Horie R, Watanabe T. CD30: expression and function in health and disease. Semin Immunol. 1998;10(6):457–70. doi:10.1006/smim.1998.0156.
Pallesen G, Hamilton-Dutoit SJ. Ki-1 (CD30) antigen is regularly expressed by tumor cells of embryonal carcinoma. Am J Pathol. 1988;133(3):446–50.
Gruss H, Boiani N, Williams D, Armitage R, Smith C, Goodwin R. Pleiotropic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood. 1994;83(8):2045–56.
Aizawa S, Nakano H, Ishida T, Horie R, Nagai M, Ito K, et al. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NFκB activation. J Biol Chem. 1997;272(4):2042–5. doi:10.1074/jbc.272.4.2042.
Mir SS, Richter BWM, Duckett CS. Differential effects of CD30 activation in anaplastic large cell lymphoma and Hodgkin disease cells. Blood. 2000;96(13):4307–12.
Ansell SM, Horwitz SM, Engert A, Khan KD, Lin T, Strair R, et al. Phase I/II Study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25(19):2764–9. doi:10.1200/jco.2006.07.8972.
Bartlett NL, Younes A, Carabasi MH, Forero A, Rosenblatt JD, Leonard JP, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111(4):1848–54. doi:10.1182/blood-2007-07-099317.
Forero-Torres A, Leonard JP, Younes A, Rosenblatt JD, Brice P, Bartlett NL, et al. A phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146(2):171–9. doi:10.1111/j.1365-2141.2009.07740.x.
Hammond PW, Vafa O, Jacinto J, Vielmetter J, Karki S, Yoder S, et al. A humanized anti-CD30 monoclonal antibody, XmAbTM2513, with enhanced in vitro potency against CD30-positive lymphomas mediated by high affinity Fc-receptor binding. Blood (ASH Annual Meeting Abstracts). 2005;106:Abstract 1470.
Blum K, Smith M, Fung H, Zalevsky J, Combs D, Ramies D, et al. (editors) Phase I study of an anti-CD30 Fc engineered humanized monoclonal antibody in Hodgkin lymphoma (HL) or anaplastic large cell lymphoma (ALCL) patients: safety, pharmacokinetics (PK), immunogenicity, and efficacy. J Clin Oncol. 2009;27(15s). Abstract 8531.
Cardarelli PM, Moldovan-Loomis M-C, Preston B, Black A, Passmore D, Chen T-H, et al. In vitro and in vivo characterization of MDX-1401 for therapy of malignant lymphoma. Clin Cancer Res. 2009;15(10):3376–83. doi:10.1158/1078-0432.ccr-08-3222.
Thertulien R, Frankel A, Evens A, Kaufman J, Horwitz S, Assad A, et al. A phase I, open-label, dose-escalation, multidose study of MDX-1401 (defucosylated human antiCD30 monoclonal antibody) in patients with CD30-positive refractory/relapsed Hodgkins lymphoma. Proceedings of the Annual Meeting of the American Association for Cancer Research; Apr 2009; Denver, CO. Philadelphia (PA): AACR; 2009. Abstract 30.
Schnell R, Dietlein M, Staak JO, Borchmann P, Schomaecker K, Fischer T, et al. Treatment of refractory Hodgkin's lymphoma patients with an iodine-131-labeled murine anti-CD30 monoclonal antibody. J Clin Oncol. 2005;23(21):4669–78. doi:10.1200/jco.2005.09.098.
Zhang M, Yao Z, Patel H, Garmestani K, Zhang Z, Talanov VS, et al. Effective therapy of murine models of human leukemia and lymphoma with radiolabeled anti-CD30 antibody, HeFi-1. Proc Natl Acad Sci USA. 2007;104(20):8444–8. doi:10.1073/pnas.0702496104.
Pasqualucci L, Wasik M, Teicher B, Flenghi L, Bolognesi A, Stirpe F, et al. Antitumor activity of anti-CD30 immunotoxin (Ber-H2/saporin) in vitro and in severe combined immunodeficiency disease mice xenografted with human CD30+ anaplastic large-cell lymphoma. Blood. 1995;85(8):2139–46.
Falini B, Flenghi L, Aversa F, Barbabietola G, Martelli M, Comeli P, et al. Response of refractory Hodgkin's disease to monoclonal anti-CD30 immunotoxin. Lancet. 1992;339(8803):1195–6.
Terenzi A, Bolognesi A, Pasqualucci L, Flenghi L, Pileri S, Stein H, et al. Anti-CD30 (BER=H2) immunotoxins containing the type-1 ribosome-inactivating proteins momordin and PAP-S (pokeweed antiviral protein from seeds) display powerful antitumour activity against CD30+ tumour cells in vitro and in SCID mice. Br J Haematol. 1996;92(4):872–9.
Bolognesi A, Tazzari PL, Olivieri F, Polito L, Falini B, Stirpe F. Induction of apoptosis by ribosome-inactivating proteins and related immunotoxins. Int J Cancer. 1996;68(3):349–55.
Schneix R, Linnartz C, Katouzi AA, Schon G, Bohlen H, Horn-Lohrens O, et al. Development of new ricin A-chain immunotoxins with potent anti-tumor effects against human hodgkin cells in vitro and disseminated Hodgkin tumors in scid mice using high-affinity monoclonal antibodies directed against the CD30 antigen. Int J Cancer. 1995;63(2):238–44. doi:10.1002/ijc.2910630216.
Bolognesi A, Tazzari PL, Legname G, Olivieri F, Modena D, Conte R, et al. Anti-CD30 immunotoxins with native and recombinant dianthin 30. Cancer Immunol Immunother. 1995;40(2):109–14. doi:10.1007/bf01520292.
Barth S, Huhn M, Matthey B, Tawadros S, Schnell R, Schinköthe T, et al. Ki-4(scFv)–ETA′, a new recombinant anti-CD30 immunotoxin with highly specific cytotoxic activity against disseminated Hodgkin tumors in SCID mice. Blood. 2000;95(12):3909–14.
Schnell R, Staak O, Borchmann P, Schwartz C, Matthey B, Hansen H, et al. A phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphoma. Clin Cancer Res. 2002;8(6):1779–86.
Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102(4):1458–65. doi:10.1182/blood-2003-01-0039.
Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21(7):778–84.
Sanderson RJ, Hering MA, James SF, Sun MMC, Doronina SO, Siadak AW, et al. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res. 2005;11(2):843–52.
• Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010;16(3):888–97. doi:10.1158/1078-0432.ccr-09-2069. This article describes the mechanism of action of brentuximab vedotin.
Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol. 2005;5(5):543–9. doi:10.1016/j.coph.2005.04.017.
• Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–21. doi:10.1056/NEJMoa1002965. First-in-human study of brentuximab vedotin.
• Fanale M, Bartlett NL, Forero-Torres A, Rosenblatt J, Horning SJ, Franklin AR, et al. The antibody-drug conjugate brentuximab vedotin (SGN-35) induced multiple objective responses in patients with relapsed or refractory CD30-positive lymphomas in a phase 1 weekly dosing study. Blood (Ash Annual Meeting Abstracts). 2009;114, abstract 2731. Safety and activity of brentuximab vedotin given in a weekly regimen.
•• Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–6. doi:10.1200/jco.2011.38.0402. Pivotal phase II trial of brentuximab vedotin in relapsed refractory sALCL.
Hombach A, Jung W, Pohl C, Renner C, Sahin U, Schmits R, et al. A CD16/CD30 bispecific monoclonal antibody induces lysis of Hodgkin's cells by unstimulated natural killer cells in vitro and in vivo. Int J Cancer. 1993;55(5):830–6.
Wu Y. Session 11: Therapeutic antibodies. Hum Antibodies. 2011;20(3–4):61–3.
Knackmuss S, Reusch U, Burkhardt C, Fucek I, Le Gall F, Pauels H-G, et al. Preclinical development of an anti-CD30/anti-CD16A bispecific tetravalent TandAb antibody for the treatment of Hodgkin lymphoma. ASCO Meeting Abstracts. 2012;30(15 suppl). e18532.
Rothe A, Younes A, Reiners KS, Dietlein M, Eichenauer DA, Kessler J, et al. A phase I study with the bispecific anti-CD30 x anti-CD16A antibody construct AFM13 in patients with relapsed or refractory hodgkin lymphoma. Blood (ASH Annual Meeting Abstracts). 2011;118(21):Abstract 3709.
Shapiro R, Riordan JF, Vallee BL. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986;25(12):3527–32. doi:10.1021/bi00360a008.
Barth S, Matthey B, Huhn M, Diehl V, Engert A. CD30L-ETA': a new recombinant immunotoxin based on the CD30 ligand for possible use against human lymphoma. Cytokines Cell Mol Ther. 1999;5(2):69–78.
Huhn M, Sasse S, Tur MK, Matthey B, Schinköthe T, Rybak SM, et al. Human angiogenin fused to human CD30 ligand (Ang-CD30L) exhibits specific cytotoxicity against CD30-positive lymphoma. Cancer Res. 2001;61(24):8737–42.
Roskrow MA, Suzuki N, Gan Y-J, Sixbey JW, Ng CYC, Kimbrough S, et al. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin's disease. Blood. 1998;91(8):2925–34.
Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med. 2004;200(12):1623–33. doi:10.1084/jem.20040890.
Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90(2):720–4.
Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Pohl C, et al. An Anti-CD30 chimeric receptor that mediates CD3-zeta-independent T-cell activation against Hodgkin's lymphoma cells in the presence of soluble CD30. Cancer Res. 1998;58(6):1116–9.
ClinicalTrials.gov. Administration of T lymphocytes for Hodgkin's lymphoma and non-Hodgkin's lymphoma (CART CD30). http://clinicaltrials.gov/ct2/show/NCT01316146. Accessed 10 Sept 2012.
Brocker T, Karjalainen K. Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J Exp Med. 1995;181(5):1653–9. doi:10.1084/jem.181.5.1653.
Brocker T. Chimeric Fv-ζ or Fv-ε receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96(5):1999–2001.
ClinicalTrials.gov. EBV CTLs expressing CD30 chimeric receptors for CD 30+ lymphoma (CARCD30). http://clinicaltrials.gov/ct2/show/NCT01192464. Accessed 10 Sept 2012.
Disclosure
J. Vadakara: none; B. Pro: consultant for Celgene, Spectrum, and Allos, pending grant/research funds from Seattle Genetics, and honoraria from Celgene and Allos.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vadakara, J., Pro, B. Targeting CD30 in Anaplastic Large Cell Lymphoma. Curr Hematol Malig Rep 7, 285–291 (2012). https://doi.org/10.1007/s11899-012-0137-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-012-0137-y