Abstract
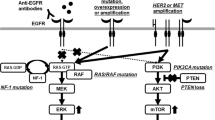
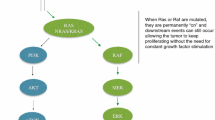
The epidermal growth factor receptor (EGFR) and its downstream signaling pathways are crucially important in the biology of colorectal cancer (CRC). In the past few years, EGFR kinase and its major downstream effector, the RAS/RAF/MAPK/ERK pathway, have been attractive targets for development of new therapy for treatment of metastatic CRC (mCRC). Cetuximab and panitumumab, two monoclonal antibodies (mAbs) against the EGFR, are the first targeted agents approved as personalized medicine for treatment of mCRC. Recently, inhibition of MEK1/2 has been seen as a promising approach for blocking MAPK-mediated proliferation signals. Several compounds with highly selective inhibitory effects on MEK1/2 have been developed and have entered clinical trials. New therapeutic agents have also been used to optimize personalized treatment of mCRC. In this context we discuss recent advances in research on EGFR and MAPK inhibitors.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA Cancer J Clin. 2014. doi:10.3322/caac.21208.
Hynes NE, Lane HA. ErbB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54.
Fry DW, Kraker AJ, McMichael A, et al. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science. 1994;265:1093–5.
Ward WHJ, Cook PN, Slater AM, et al. Epidermal growth factor receptor tyrosine kinase. Investigation of catalytic mechanism, structure-based searching and discovery of a potent inhibitor. Biochem Pharmacol. 1994;48:659–66.
Rodríguez J, Viúdez A, Ponz-Sarvisé M, et al. Improving disease control in advanced colorectal cancer: panitumumab and cetuximab. Crit Rev Oncol Hematol. 2010;74:193–202.
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45.
Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71.
Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8.
Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64.
Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–705. The authors emphasize the importance of KRAS testing for patients with mCRC.
Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–13.
Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–61.
Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22.
Bamford S, Dawson E, Forbes S, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91:355–8.
Yarden Y. The EGFR family and its ligands in human cancer signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37:S3–8.
Ciardiello F, Tortora G. EGFR antagonist in cancer treatment. N Engl J Med. 2008;358:1160–74.
Baselga J, Pfister D, Cooper MR, et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18:904–14.
Robert F, Ezekiel MP, Spencer SA, et al. Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–43.
Foon KA, Yang XD, Weiner LM, et al. Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys. 2004;58:984–90.
Normanno N, Bianco C, De Luca A, et al. Target-based agents against ErbB receptors and their ligands: a novel approach to cancer treatment. Endocrinol Relat Cancer. 2003;10:1–21.
Saltz L, Meropol NJ, Loehrer PJ, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that express the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8.
Lenz HJ, Van Cutsem E, Khambata-Ford S, et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006;24:4914–21.
Sobrero AF, Maurel J, Fehrenbacher L. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–9.
Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–14.
Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755–62. In this trial the authors showed that cetuximab did not add significant benefit to the FLOX regimen for first-line treatment of mCRC.
Morgillo F, Cantile F, Fasano M, et al. Resistance mechanisms of tumour cells to EGFR inhibitors. Clin Transl Oncol. 2009;11:270–5.
Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5.
Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–8.
Van Cutsem E Lang I, Folprecht G, et al. Cetuximab plus FOLFIRI in the treatment of metastatic colorectal cancer (mCRC): The influence of KRAS and BRAF biomarkers on outcome: Updated data from the CRYSTAL trial. Presented at the 2010 Gastrointestinal Cancers Symposium, Jan 22-24, Orlando, FL. Abstract 281.
Kohne C, Rogier P, Stroh C. Cetuximab with chemotherapy (CT) as first-line treatment for metastatic colorectal cancer (mCRC): A meta-analysis of the CRYSTAL and OPUS studies according to KRAS and BRAF mutation status. Presented at the 2010 Gastrointestinal Cancers Symposium, Jan 22-24, Orlando, FL. Abstract 406.
Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–34. In this trial the authors emphasize the importance of better selection of patients who might benefit from anti-EGFR moAbs by considering other RAS mutations.
Stintzing S, Jung A, Rossius L, et al. Analysis of KRAS/NRAS and BRAF mutations in FIRE-3: A randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients. ESMO 2013, Amsterdam, The Netherlands: abstract LBA 17. This trial emphasizes the importance of RAS analysis and is the first randomised multicenter study to directly compare two biological agents that are active in mCRC.
Ciardiello F, Normanno N, Maiello E, et al. Molecular profiling of the CAPRI GOIM trial in KRAS wild type (wt) metastatic colorectal cancer (mCRC) patients (pts): Cetuximab + FOLFIRI followed by FOLFOX4 ± cetuximab. ESMO 2013. Amsterdam, The Netherlands: abstract LBA 31. The authors report high intra and inter-tumor heterogeneity, indicating that several mutations can coexist in the same cancer cell, and, by considering RAS mutations, identify a subgroup of patients most likely to benefit from FOLFIRI plus cetuximab in first-line treatment.
Yang XD, Jia XC, Corvalan JR, et al. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol. 2001;38:1723.
Peeters M, Price T, Hotko Y, et al. Randomized phase III study (20050181) of panitumumab with FOLFIRI compared to FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer (mCRC). Eur J Cancer. 2009;7:9.
European Medicines Agency: Committee for Medicinal Products for Human Use. May 2008 plenary meeting monthly report. http://www.emea.europa.eu/pdfs/human/press/pr/27923508en.pdf.
Genome Web Daily News. FDA adds KRAS testing info to Vectibix, Erbitux Labels (Press Release, Genome Web News). http://www.genomeweb.com/dxpgx/fda-adds-kras-testing-info-vectibix-erbituxlabels.
Friedman LM, Rinon A, Schechter B, et al. Synergistic down regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc Natl Acad Sci U S A. 2005;102:1915–20.
Kamat V, Donaldson JM, Kari C, et al. Enhanced EGFR inhibition and distinct epitope recognition By EGFR antagonistic MABS C225 and 425. Cancer Biol Ther. 2008;7:726–33.
Pedersen MW, Jacobsen HJ, Koefoed K, et al. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res. 2010;70:588–97.
Skartved NJ, Jacobsen HJ, Pedersen MW, et al. Preclinical pharmacokinetics and safety of Sym004: a synergistic antibody mixture directed against epidermal growth factor receptor. Clin Cancer Res. 2011;17:5962–72.
Iida M, Brand TM, Starr MM, et al. Sym004, a novel EGFR antibody mixture, can overcome acquired resistance to cetuximab. Neoplasia. 2013;15:1196–206.
Tabernero et al. An Open-label, Multi-center Phase I Dose Escalation Study to Investigate the Safety and Tolerability of Multiple Doses of Sym004 in Patients With Advanced Solid Tumors. ClinicalTrials.gov Identifier: NCT01117428.
Gerdes CA, Nicolini V, Herter S, et al. GA201 (RG7160): a novel, humanized, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab. Clin Cancer Res. 2013;19:1126–38.
Delord JP, Tabernero J, Garcıa-Carbonero R, et al. Open-label, multicentre expansion cohort to evaluate imgatuzumab in pre-treated patients with KRAS-mutant advanced colorectal carcinoma. Eur J Cancer. 2014;50:496–505.
Paz Ares L, Gomez Roca C, Delord JP, et al. Phase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor (EGFR) in patients with advanced solid tumors. J Clin Oncol. 2011;29:3783–90.
Cervantes-Ruiperez A, Markman B, Siena S. et al. The GAIN-C study (BP25438): Randomized phase II trial of RG7160 (GA201) plus FOLFIRI, compared to cetuximab plus FOLFIRI or FOLFIRI alone in second-line KRAS wild type (WT) or mutant metastatic colorectal cancer (mCRC). ASCO Annual Meeting. 2012. J Clin Oncol 30, 2012abstr: TPS3637A).
Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342–6.
Akinleye A, Furqan M, Mukhi N, et al. MEK and the inhibitors: from bench to bedside. J Hematol Oncol. 2013;6:27.
Maekawa M, Nishida E, Tanoue T. Identification of the anti-proliferative protein Tob as a MAPK substrate. J Biol Chem. 2002;277:37783–7.
Roux PP, Ballif BA, Anjum R, et al. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–94.
Ledwith BJ, Manam S, Kraynak AR, et al. Antisense-fos RNA causes partial reversion of the transformed phenotypes induced by the c-Ha-ras oncogene. Mol Cell Biol. 1990;10:1545–55.
Sebolt-Leopold JS. MEK inhibitors: a therapeutic approach to targeting the Ras-MAP kinase pathway in tumors. Curr Pharm Des. 2004; 1907–14.
Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417949–54.
Marks JL, Gong Y, Chitale D, et al. Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res. 2008;68:5524–8.
Allen LF, Sebolt-Leopold JS, Meyer MB. CI-1040 (PD-184,352), a targeted signal transduction inhibitor of MEK (MAPKK). Semin Oncol. 2003;30:105–16.
Lorusso PM, Adjei AA, Varterasian M, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. JCO. 2005;23:5281–93.
Rinehart J, Adjei AA, Lorusso PM. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non–small-cell Lung, Breast, Colon, and Pancreatic cancer. J Clin Oncol. 2004;22:4456–62.
Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY- 142886), a potent inhibitor of mitogen-activated protein kinase/ extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–19.
Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–83.
Troiani T, Vecchione L, Martinelli E, et al. Intrinsic resistance to selumetinib, a selective inhibitor of MEK1/2, by cAMP-dependent protein kinase A activation in human lung and colorectal cancer cells. Br J Cancer. 2012;106:1648–59.
Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small- molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–46.
Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res. 2012;18:555–67.
Hainsworth JD, Cebotaru CL, Kanarev V, et al. A phase II, open-label, randomized study to assess the efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens. J Thorac Oncol. 2010;5:1630–6.
Bodoky G, Timcheva C, Spigel DR, et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Investig New Drugs. 2012;30:1216–23.
Bennouna J, Lang I, Valladares-Ayerbes M, et al. A Phase II, open-label, randomized study to assess the efficacy and safety of the MEK1/2 inhibitor AZD6244 (ARRY-142886) versus capecitabine monotherapy in patients with colorectal cancer who have failed one or two prior chemotherapeutic regimens. Investig New Drugs. 2011;29:1021–8.
Yoon J, Koo KH, Choi KY. MEK1/2 inhibitors AS703026 and AZD6244 may be potential therapies for KRAS mutated colorectal cancer that is resistant to EGFR monoclonal antibody therapy. Cancer Res. 2011;71:445–53.
SchelmanW et al. Selumetinib and Cetuximab in Treating Patients With Refractory Solid Tumors. ClinicalTrials.gov identifier: NCT01217450.
Azad N et al. Selumetinib and Cixutumumab in Treating Patients With Advanced Solid Malignancies. ClinicalTrials.gov Identifier: NCT01061749.
Iverson C, Larson G, Lai C, et al. RDEA119/BAY 869766: a potent, selective, allosteric inhibitor of MEK1/2 for the treatment of cancer. Cancer Res. 2009;69:6839–47.
Weekes CD, Von Hoff DD, Adjei AA, et al. Multicenter phase I trial of the mitogen-activated protein kinase 1/2 inhibitor BAY 86-9766 in patients with advanced cancer. Clin Cancer Res. 2013;19:1232–43.
Chang Q, Chapman MS, Miner JN, et al. Antitumor activity of a potent MEK inhibitor RDEA119/BAY 869766 combined with rapamycin in human orthotopic primary pancreatic cancer xenografts. BMC Cancer. 2010;10:515.
Infante JR, Fecher LA, Nallapareddy S, et al. Safety and efficacy results from the first-in-human study of the oral MEK 1/2 inhibitor GSK1120212. J Clin Oncol. 2010;28 abstr:2503.
Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773–81.
Flaherty KT, Fecher LA, Falchook GS, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14. The authors showed that trametinib improved progression-free and overall survival of patients with advanced melanoma.
Wuthrick E et al. Trametinib, Fluorouracil, and Radiation Therapy Before Surgery in Treating Patients With Stage II-III Rectal Cancer.ClinicalTrials.gov Identifier: NCT0174064
Houede N, Faivre SJ, Awada A, et al. Safety and evidence of activity of MSC1936369, an oral MEK1/2 inhibitor, in patients with advanced malignancies. J Clin Oncol. 2011; 2915s, abstr 3019.
Macarulla T, Cervantes A, Roselló S, et al. Phase i/ii study of folfiri plus the mek1/2 inhibitor pimasertib (msc1936369b) as second-line treatment for kras mutated metastatic colorectal cancer. Ann Oncol. 2012;23 suppl 4:iv19–30.
Sidhu SS, Egile C, Malfilatre M et al. Anti-tumor activity of pimasertib in combination with SAR245409 or SAR245408 in human primary colorectal cancer xenograft models bearing PI3K/KRas and KRas mutations. Cancer Research. 2013; Volume 73, Issue 8, Supplement 1 doi: 10.1158/1538-7445.AM2013-4638, AACR 104th Annual Meeting 2013; Apr 6-10; Washington, DC. Abstract 4638
Martinelli E, Troiani T, D’Aiuto E. Antitumor activity of pimasertib, a selective MEK 1/2 inhibitor, in combination with PI3K/mTOR inhibitors or with multi-targeted kinase inhibitors in pimasertib-resistant human lung and colorectal cancer cells. Int J Cancer. 2013;133:2089–101.
Ishii N, Harada N, Joseph EW, et al. Enhanced inhibition of ERK signaling by a novel allosteric MEK inhibitor, CH5126766, that suppresses feedback reactivation of RAF activity. Cancer Res. 2013;73:4050–60.
Little AS, Balmanno K, Sale MJ, et al. Amplification of the driving oncogene, KRAS or BRAF, underpins acquired resistance to MEK1/2 inhibitors in Colorectal Cancer cells. Sci Signal. 2011;4:170.
Martinez-Garcia M, Banerji U, Albanell J, et al. First-in-human, phase I dose-escalation study of the safety pharmacokinetics, and pharmacodynamics of RO5126766, a first-in-class dual MEK/RAF inhibitor in patients with solid tumors. Clin Cancer Res. 2012;18:4806–19.
Troiani T, Zappavigna S, Martinelli E, et al. Optimizing treatment of metastatic colorectal cancer patients with anti-EGFR antibodies: overcoming the mechanisms of cancer cell resistance. Expert Opin Biol Ther. 2013;13:241–55.
De Roock W, De Vriendt V, Nomanno N, et al. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011;12:594–603. The authors show that other activating RAS mutations predict poorer outcome for mCRC patients treated with both cetuximab and panitumumab.
Compliance with Ethics Guidelines
Conflict of Interest
Erika Martinelli, Stefania Napolitano, Davide Ciardiello, Fortunato Ciardiello, and Teresa Troiani declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinelli, E., Napolitano, S., Ciardiello, D. et al. Optimization of the Development of Old and New EGFR and MAP Kinase Inhibitors for Colorectal Cancer. Curr Colorectal Cancer Rep 10, 279–287 (2014). https://doi.org/10.1007/s11888-014-0233-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-014-0233-6